685836
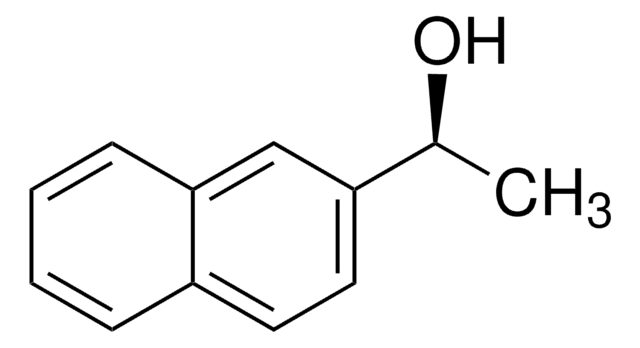
(S)-(−)-1-Phenylethanol
97%
Synonym(s):
(−)-Methyl phenyl carbinol, (S)-(−)-α-Methylbenzyl alcohol, (S)-(−)-sec-Phenylethyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H10O
CAS Number:
Molecular Weight:
122.16
Beilstein:
2039797
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
optical activity
[α]22/D -44.0°, neat
bp
88-89 °C/10 mmHg (lit.)
mp
9-11 °C (lit.)
density
1.012 g/mL at 20 °C (lit.)
functional group
hydroxyl
phenyl
SMILES string
C[C@H](O)c1ccccc1
InChI
1S/C8H10O/c1-7(9)8-5-3-2-4-6-8/h2-7,9H,1H3/t7-/m0/s1
InChI key
WAPNOHKVXSQRPX-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(S)-(-)-1-Phenylethanol can be prepared from acetophenone via bioreduction in the presence of Rhizopus arrhizus as a biocatalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Laboratory scale-up synthesis of chiral carbinols using Rhizopus arrhizus.
Salvi NA and Chattopadhyay S.
Tetrahedron Asymmetry, 27(4), 188-192 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service