All Photos(1)
About This Item
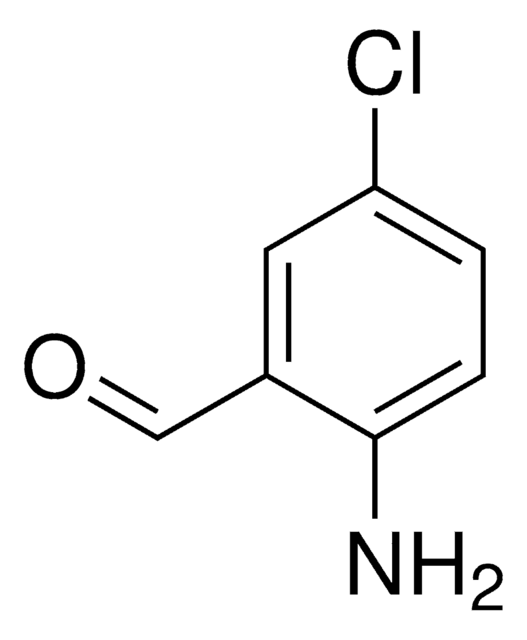
Linear Formula:
H2NC6H2(Br)2CHO
CAS Number:
Molecular Weight:
278.93
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
130-135 °C (lit.)
SMILES string
Nc1c(Br)cc(Br)cc1C=O
InChI
1S/C7H5Br2NO/c8-5-1-4(3-11)7(10)6(9)2-5/h1-3H,10H2
InChI key
RCPAZWISSAVDEA-UHFFFAOYSA-N
General description
2-Amino-3,5-dibromobenzaldehyde has been identified as one of the oxidation product of bromhexine by controlled potential electrolysis followed by HPLC-UV and GC-MS. It participates in the Friedländer condensation of C-β−glycosylic ketones to form 2-substituted quinoline derivatives.
Application
2-Amino-3,5-dibromobenzaldehyde may be employed in the preparation of tetradentate Schiff base ligands, via condensation with aliphatic diamines. These ligands forms nickel (II) and oxovanadium(IV) complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Subbiah Nagarajan et al.
Carbohydrate research, 345(14), 1988-1997 (2010-08-07)
Regioselective facile one-pot synthesis of 16 different sugar-based quinoline, naphthyridine, and xanthone derivatives is reported. The compounds are characterized by NMR spectroscopy and elemental analysis. The beta-Anomeric form of the sugar moiety was identified from (1)H NMR studies. Antimicrobial studies
Khosro Mohammadi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 146, 221-227 (2015-03-31)
The tetradentate Schiff base ligands (L(1)-L(4)), were synthesized by reaction between 2-amino-3,5-dibromobenzaldehyde and aliphatic diamines. Then, nickel and oxovanadium(IV) complexes of these ligands were synthesized and characterized by (1)H NMR, Mass, IR, UV-Vis spectroscopy and thermogravimetry. The kinetic parameters of
M Turchán et al.
Talanta, 73(5), 913-919 (2007-10-31)
A complete electrochemical study and a novel electroanalytical procedure for bromhexine quantitation are described. Bromhexine in methanol/0.1molL(-1) Britton-Robinson buffer solution (2.5/97.5) shows an anodic response on glassy carbon electrode between pH 2 and 7.5. By DPV and CV, both peak
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![4-[(2-Cyanoethyl)methylamino]benzaldehyde 98%](/deepweb/assets/sigmaaldrich/product/structures/205/945/c1d5652c-9386-4c27-9f59-6ba6e104181b/640/c1d5652c-9386-4c27-9f59-6ba6e104181b.png)