All Photos(1)
About This Item
Empirical Formula (Hill Notation):
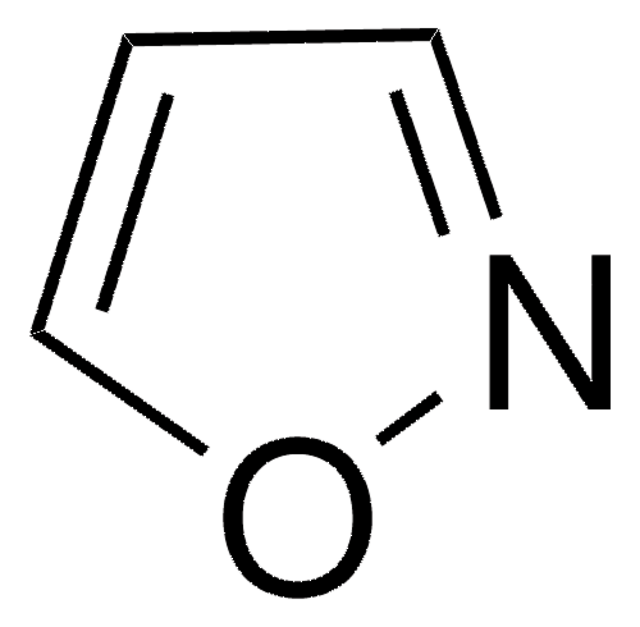
C3H3NO
CAS Number:
Molecular Weight:
69.06
Beilstein:
103851
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.425 (lit.)
bp
69-70 °C (lit.)
mp
−87-−84 °C (lit.)
density
1.05 g/mL at 25 °C (lit.)
SMILES string
c1cocn1
InChI
1S/C3H3NO/c1-2-5-3-4-1/h1-3H
InChI key
ZCQWOFVYLHDMMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Oxazole is the parent molecule for a large class of heterocyclic aromatic compounds. It is a weak base that can be used as an electron-deficient diene in the Diels-Alder cycloaddition reaction. It undergoes nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and acylation.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
66.2 °F - closed cup
Flash Point(C)
19 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Haseen Ahmad et al.
European journal of medicinal chemistry, 208, 112759-112759 (2020-09-05)
Oxazole derivatives are important medicinal compounds which are inhibitors of various enzymes such as NPP1, NPP2, NPP3, tyrosine kinase, dipeptidyl-peptidase IV, cyclooxygenase-2, and protein tyrosine phosphatase. In this study, an extensive range of new biologically active biphenyl oxazole derivatives was
Maryna V Kachaeva et al.
Computational biology and chemistry, 74, 294-303 (2018-04-27)
Based on modern literature data about biological activity of E7010 derivatives, a series of new sulfonamides as potential anticancer drugs were rationally designed by QSAR modeling methods Сlassification learning QSAR models to predict the tubulin polymerization inhibition activity of novel
Bo Pang et al.
Journal of the American Chemical Society, 142(25), 10931-10935 (2020-06-09)
Nonribosomal peptide synthetase (NRPS) oxidase (Ox) domains oxidize protein-bound intermediates to install crucial structural motifs in bioactive natural products. The mechanism of this domain remains elusive. Here, by studying indigoidine synthetase, a single-module NRPS involved in the biosynthesis of indigoidine
Lori M Culberson et al.
Physical chemistry chemical physics : PCCP, 16(9), 3964-3972 (2014-01-22)
Bond breaking is a challenging problem in both experimental and theoretical chemistry, due to the transient nature and multi-configurational electronic structure of dissociating molecules. We use anion photodetachment to probe the diradical interactions in the ring-opening reaction of oxazole and
Systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals: A review
Joshi S, et al.
The pharma innovation journal, 6, 109-109 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service