All Photos(1)
About This Item
Linear Formula:
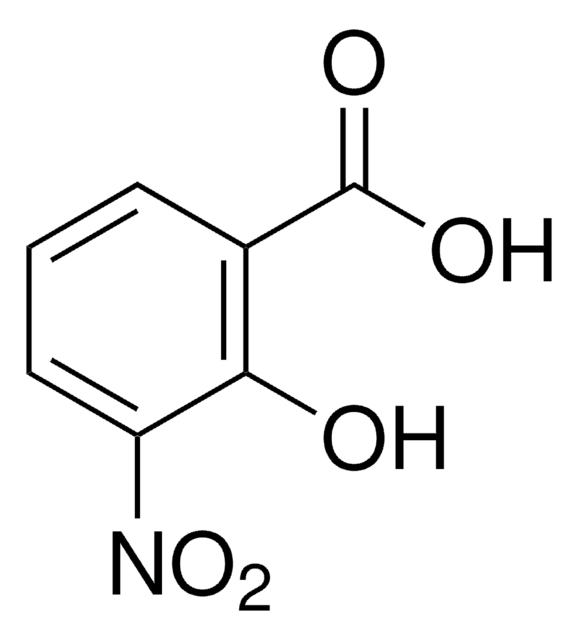
O2NC6H4CH2CO2H
CAS Number:
Molecular Weight:
181.15
Beilstein:
1959243
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
137-140 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)Cc1ccccc1[N+]([O-])=O
InChI
1S/C8H7NO4/c10-8(11)5-6-3-1-2-4-7(6)9(12)13/h1-4H,5H2,(H,10,11)
InChI key
WMUZDBZPDLHUMW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Nitrophenylacetic acid was used as an internal standard in the determination of the theophylline solubilizer salicylamide-O-acetic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M K Danks et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 5(4), 917-924 (1999-04-23)
Several recent studies have examined the possibility of producing tumor-specific cytotoxicity with various enzyme/ prodrug combinations. The enzymes are targeted to tumor cells either with antibodies (ADEPT, antibody directed enzyme prodrug therapy) or with viruses (VDEPT). The goal of the
H U Schulz et al.
Journal of pharmaceutical and biomedical analysis, 3(5), 469-475 (1985-01-01)
A high-performance liquid chromatographic method for the determination of the theophylline solubilizer salicylamide-O-acetic acid has been developed in the range 0.5 to 10 microg/ml for human serum and 5 to 400 microg/ml for urine. Reversed-phase ion-pair chromatography was employed with
Leveraging a small-molecule modification to enable the photoactivation of rho GTPases.
Katryn R Harwood et al.
Chembiochem : a European journal of chemical biology, 10(18), 2855-2857 (2009-10-31)
Manuela F Frasco et al.
The FEBS journal, 274(7), 1849-1861 (2007-03-16)
The poorly known mechanism of inhibition of cholinesterases by inorganic mercury (HgCl2) has been studied with a view to using these enzymes as biomarkers or as biological components of biosensors to survey polluted areas. The inhibition of a variety of
Jan-Michael Mewes et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(11), 2077-2080 (2011-06-10)
Femtosecond spectroscopy and quantum chemical calculations provide detailed insights into the specificities of the uncaging mechanism of CO2 from ortho-, meta-, and para-nitrophenylacetate. The emerging general principles allow a rational design of improved ortho-nitrophenyl cages for chemical and biological applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service