T1783
Tobramycin sulfate salt
aminoglycoside antibiotic
Synonyme(s) :
Nebramycin Factor 6 sulfate
About This Item
Produits recommandés
Source biologique
Streptomyces tenebrarius
Forme
powder
Puissance
634-739 μg per mg
Conditions de stockage
(Keep container tightly closed in a dry and well-ventilated place. Hygroscopic.)
Couleur
white to off-white
Solubilité
H2O: soluble 50 mg/mL
Spectre d'activité de l'antibiotique
Gram-negative bacteria
Mode d’action
protein synthesis | interferes
Température de stockage
2-8°C
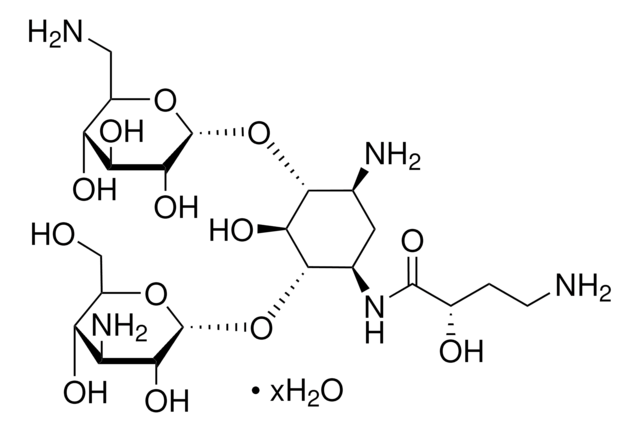
Chaîne SMILES
OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](N)C[C@@H]1O
InChI
1S/C18H37N5O9.H2O4S/c19-3-9-8(25)2-7(22)17(29-9)31-15-5(20)1-6(21)16(14(15)28)32-18-13(27)11(23)12(26)10(4-24)30-18;1-5(2,3)4/h5-18,24-28H,1-4,19-23H2;(H2,1,2,3,4)/t5-,6+,7+,8-,9+,10+,11-,12+,13+,14-,15+,16-,17+,18+;/m0./s1
Clé InChI
ZEUUPKVZFKBXPW-TWDWGCDDSA-N
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Mode of Action: Binds to 70S ribosomal subunit; inhibits translocation; elicits miscoding.
Spectrum of Activity: Gram negative bacteria. Not effective against Enterococci.
Conditionnement
Attention
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Repr. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique