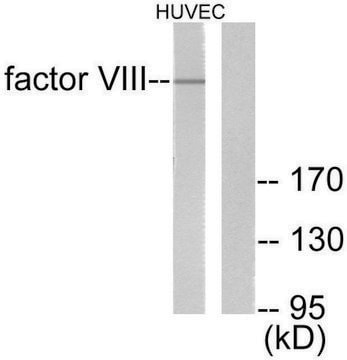
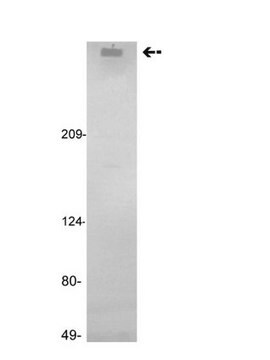
H0920000
Human coagulation factor VIII concentrate
European Pharmacopoeia (EP) Reference Standard
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Fabricant/nom de marque
EDQM
Application(s)
pharmaceutical (small molecule)
Format
neat
Description générale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Human coagulation factor VIII concentrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Conditionnement
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Autres remarques
Sales restrictions may apply.
Clause de non-responsabilité
Ce produit, destiné à la recherche scientifique, est soumis à une réglementation spécifique en France, y compris pour les activités d′importation et d′exportation (Article L 1211-1 alinéa 2 du Code de la Santé Publique). L′acheteur (c′est-à-dire l′utilisateur FINAL) est tenu d′obtenir une autorisation d′importation auprès du ministère français de la recherche, mentionné à l′article L1245-5-1 II du Code de la Santé Publique. En commandant ce produit, vous confirmez détenir l′autorisation d′importation requise.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Puneet Gaitonde et al.
The Journal of biological chemistry, 288(24), 17051-17056 (2013-05-08)
Administration of recombinant factor VIII (FVIII), an important co-factor in blood clotting cascade, elicits unwanted anti-FVIII antibodies in hemophilia A (HA) patients. Previously, FVIII associated with phosphatidylserine (PS) showed significant reduction in the anti-FVIII antibody response in HA mice. The
Innovations in coagulation: improved options for treatment of hemophilia A and B.
Ingrid Pabinger-Fasching et al.
Thrombosis research, 131 Suppl 2, S1-S1 (2013-03-30)
Samantha C Gouw et al.
Blood, 121(20), 4046-4055 (2013-04-05)
The objective of this study was to examine the association of the intensity of treatment, ranging from high-dose intensive factor VIII (FVIII) treatment to prophylactic treatment, with the inhibitor incidence among previously untreated patients with severe hemophilia A. This cohort
Stefan Schulte
Thrombosis research, 131 Suppl 2, S2-S6 (2013-03-30)
Albumin fusion technology has been used to enhance the pharmacokinetic properties of recombinant coagulation factors. The goal of linking albumin to coagulation factors is to extend the half-life of the coagulation factor, thereby allowing for less frequent dosing for patients
S V Ignat'ev et al.
Klinicheskaia laboratornaia diagnostika, (3)(3), 20-22 (2013-07-03)
The sample consisted of 102 patients with hemophilia infected and non-infected with hepatitis viruses. It is established that in case of inhibitory form of hemophilia concentration of IgG increases at the expense of subclass II and in case of non-inhibitory
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique