N-046
(+)-Norpseudoephedrine hydrochloride solution
100 μg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
Synonyme(s) :
Cathine hydrochloride
About This Item
Produits recommandés
Qualité
certified reference material
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIB (Portugal)
Concentration
100 μg/mL in methanol (as free base)
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
forensics and toxicology
Format
single component solution
Température de stockage
−20°C
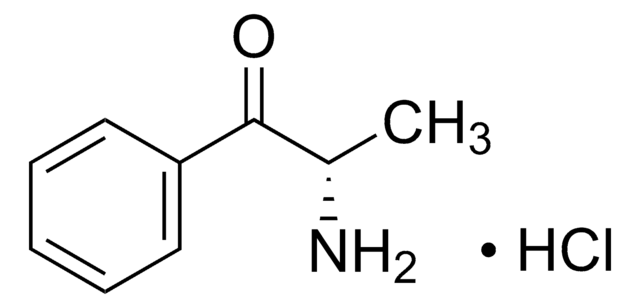
Chaîne SMILES
Cl.C[C@H](N)[C@@H](O)c1ccccc1
InChI
1S/C9H13NO.ClH/c1-7(10)9(11)8-5-3-2-4-6-8;/h2-7,9,11H,10H2,1H3;1H/t7-,9+;/m0./s1
Clé InChI
DYWNLSQWJMTVGJ-DKXTVVGFSA-N
Description générale
Application
- Phytochemical Profiling and Therapeutic Activities: (+)-Norpseudoephedrine hydrochloride solution is utilized in phytochemical profiling studies, specifically analyzing the toxicity, wound healing, analgesic, and anti-inflammatory properties of Musa paradisiaca L. stem extracts. This research enhances the understanding of natural products in pharmaceutical applications, particularly for their therapeutic potential (Ekweogu et al., 2024).
- Chiral Analysis of Stimulant Drugs: The solution is critical in the development and application of a chiral high-performance liquid chromatography-tandem mass spectrometry method for forensic analysis. This method is used to determine the presence of related amphetamine-type stimulants, providing essential data for law enforcement and drug regulation agencies (Schwelm et al., 2020).
- Microbial Production of Phenylalkylamines: (+)-Norpseudoephedrine hydrochloride serves as a biochemical tool in studies focused on the microbial production of ephedrine and pseudoephedrine, revealing significant implications for the manufacturing of pharmaceuticals and enhancing our understanding of metabolic pathways (Morris et al., 2018).
- Improved Detection Methods for Cathinones: This compound is integral to the improved gas chromatography method for detecting active principles in Catha edulis, demonstrating its utility in identifying and quantifying natural sources of cathinones, which are important for both medical research and drug enforcement (Dell′acqua et al., 2013).
- Chiral Capillary Electrophoresis: Used in chiral capillary electrophoresis for methamphetamine profiling, (+)-Norpseudoephedrine hydrochloride solution assists in distinguishing isomers in forensic samples, which is critical for the precise identification of controlled substances in forensic settings (Iwata et al., 2013).
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique