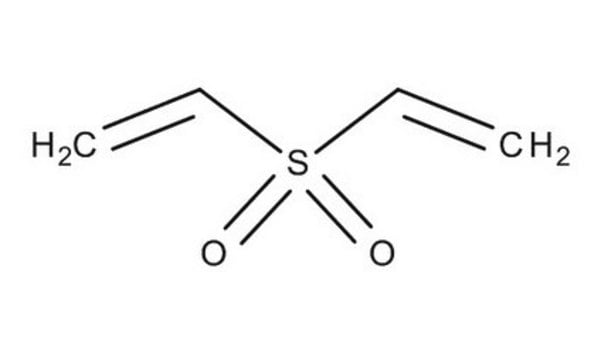
V3700
Divinyl sulfone
contains hydroquinone as inhibitor, ≥96%
Synonyme(s) :
Vinyl sulfone
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥96%
Contient
hydroquinone as inhibitor
Indice de réfraction
n20/D 1.476 (lit.)
Point d'ébullition
234 °C (lit.)
Pf
−26 °C (lit.)
Densité
1.177 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
C=CS(=O)(=O)C=C
InChI
1S/C4H6O2S/c1-3-7(5,6)4-2/h3-4H,1-2H2
Clé InChI
AFOSIXZFDONLBT-UHFFFAOYSA-N
Informations sur le gène
human ... LOC129293(129293)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- A cross-linking agent to synthesize divinyl sulfone-crosslinked hyaluronic acid hydrogels for specific biomedical applications, such as tissue engineering or drug delivery.
- A cross-linking agent to develop the conducting polymer film with MXene layers. This crosslinking can enhance the mechanical properties and stability of the composite film.
DVS and its mono and di-substituted derivatives are useful starting materials in the preparation of thiomorpholine 1,1-dioxides and other synthetically important macro- and the heterocycles.
DVS may be used to shrink proofing cotton by crosslinking it with cellulose.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
215.6 °F - closed cup
Point d'éclair (°C)
102 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique