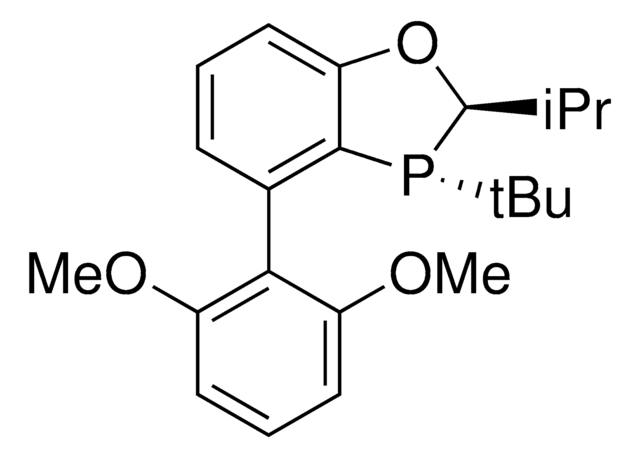
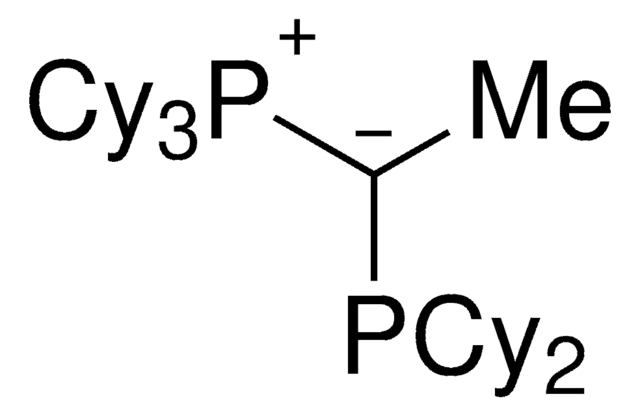
912042
joYPhos™
Umicore
Synonyme(s) :
(CyYPhos)(Ph)PCy2, Tricyclohexyl((dicyclohexyl-phosphanyl)(phenyl)methylene)-phosphane
About This Item
Produits recommandés
Niveau de qualité
Forme
powder
Pertinence de la réaction
reagent type: ligand
Pf
205-214 °C
Groupe fonctionnel
phosphine
Description générale
Application
Learn more about ylide-functionalized phosphines (YPhos)
Caractéristiques et avantages
Informations légales
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Electron-rich ylide-substituted phosphine ligands allow for palladium catalyzed coupling reactions at mild reaction conditions. These YPhos ligands enable the conversion of aryl chlorides with short reaction times.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique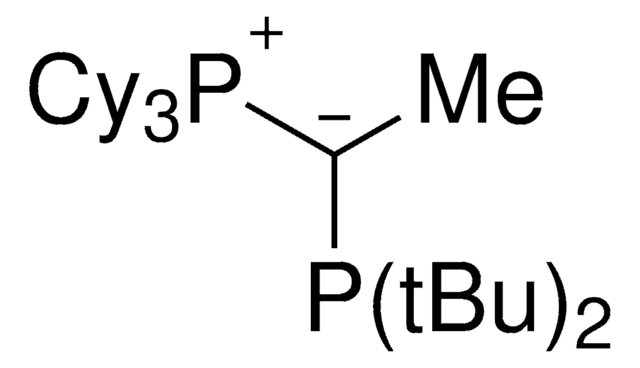

![5-(Di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/137/599/8b2f4b58-3384-40aa-9295-0887f7985525/640/8b2f4b58-3384-40aa-9295-0887f7985525.png)