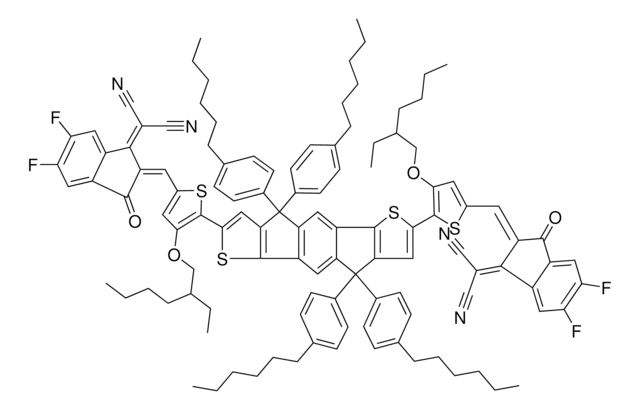
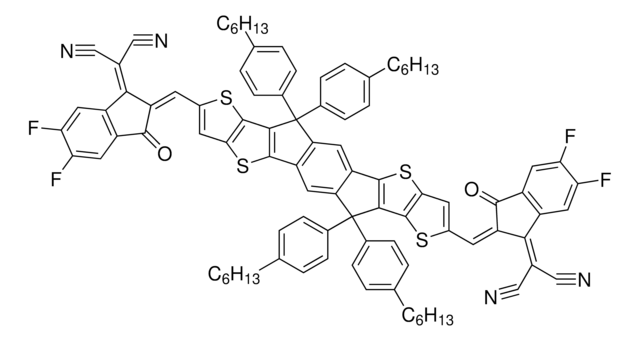
772372
DTS(PTTh2)2
Synonyme(s) :
4,4′-[4,4-Bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b′]dithiophene-2,6-diyl]bis[7-(5′-hexyl-[2,2′-bithiophen]-5-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine], 5,5′-Bis{[4-(7-hexylthiophen-2-yl)thiophen-2-yl]-[1,2,5]thiadiazolo[3,4-c]pyridine}-3,3′-di-2-ethylhexylsilylene-2,2′-bithiophene
About This Item
Produits recommandés
Forme
solid
Niveau de qualité
Pf
208-213 °C
Solubilité
chloroform: soluble(lit.)
dichlorobenzene: soluble(lit.)
λmax
655 nm in chloroform
Chaîne SMILES
CCC(CCCC)C[Si]1(CC(CCCC)CC)C2=C(SC(C3=NC=C(C4=CC=C(C5=CC=C(CCCCCC)S5)S4)C6=NSN=C63)=C2)C7=C1C=C(C8=NC=C(C9=CC=C(C%10=CC=C(CCCCCC)S%10)S9)C%11=NSN=C%118)S7
InChI
1S/C62H72N6S8Si/c1-7-13-17-19-23-41-25-27-47(69-41)49-31-29-45(71-49)43-35-63-57(59-55(43)65-75-67-59)51-33-53-61(73-51)62-54(77(53,37-39(11-5)21-15-9-3)38-40(12-6)22-16-10-4)34-52(74-62)58-60-56(66-76-68-60)44(36-64-58)46-30-32-50(72-46)48-28-26-42(70-48)24-20-18-14-8-2/h25-36,39-40H,7-24,37-38H2,1-6H3
Clé InChI
NOJURONZIGXBEP-UHFFFAOYSA-N
Description générale
Application
OPV Device Structure: ITO/MoOx/DTS(PTTh2)2: PC70BM/Al
- JSC = 14.4 mA/cm2
- VOC = 0.78 V
- FF = 0.59
- PCE = 6.7%
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![[6,6]-Phenyl C71 butyric acid methyl ester 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)


![Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-bithiophene] 99.9%](/deepweb/assets/sigmaaldrich/product/structures/309/000/8b4a3f54-7765-4aca-96c4-74ce328d455d/640/8b4a3f54-7765-4aca-96c4-74ce328d455d.png)