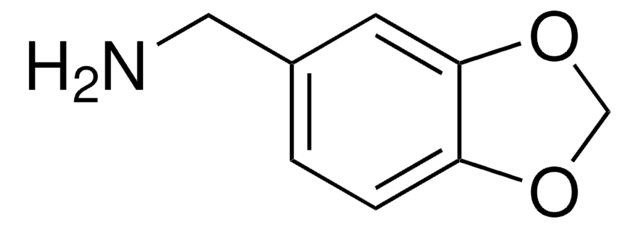
560529
3,4-Methylenedioxyphenethylamine hydrochloride
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C9H11NO2 · HCl
Numéro CAS:
Poids moléculaire :
201.65
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
98%
Pf
216-218 °C (lit.)
Groupe fonctionnel
amine
Chaîne SMILES
Cl.NCCc1ccc2OCOc2c1
InChI
1S/C9H11NO2.ClH/c10-4-3-7-1-2-8-9(5-7)12-6-11-8;/h1-2,5H,3-4,6,10H2;1H
Clé InChI
NDYXFQODWGEGNU-UHFFFAOYSA-N
Description générale
3,4-Methylenedioxyphenethylamine hydrochloride can be synthesized by reacting aluminum chloride, LiAlH4 and 3,4-methylenedioxyphenylacetonitrile.
Application
3,4-Methylenedioxyphenethylamine hydrochloride may be used to synthesize N-(3,4-methylenedioxyphenethyl)-2-(3-bromo-4-methoxyphenyl)acetamide.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
D J De Silva et al.
Neuroscience, 134(4), 1363-1375 (2005-08-02)
Substituted amphetamines such as p-chloroamphetamine and the abused drug methylenedioxymethamphetamine cause selective destruction of serotonin axons in rats, by unknown mechanisms. Since some serotonin neurones also express neuronal nitric oxide synthase, which has been implicated in neurotoxicity, the present study
An aryne route to laureline, and related topics.
Gibson MS, et al.
J. Chem. Soc. Sect. C, 16, 2234-2238 (1970)
Jan G Bruhn et al.
Journal of psychoactive drugs, 40(2), 219-222 (2008-08-30)
Human interest in psychoactive phenethylamines is known from the use of mescaline-containing cacti and designer drugs such as Ecstasy. From the alkaloid composition of cacti we hypothesized that substances resembling Ecstasy might occur naturally. In this article we show that
Milica Ninković et al.
Nephrology (Carlton, Vic.), 13(1), 33-37 (2008-01-18)
The mechanism of MDMA (3,4-methylenedioxymethamphetamine)-induced toxicity is believed to be, in part, due to enhanced oxidative stress. As MDMA is eliminated via the kidney, the aim of this study was to investigate whether MDMA created conditions of oxidative stress within
J F Bagli et al.
Journal of medicinal chemistry, 19(7), 876-882 (1976-07-01)
Synthesis of a series of thienylethanolamines having varying substituents on the thiophene ring and on the nitrogen atom is described using the general procedure reported earlier. In the determination of their pharmacological profile, some of the derivatives showed marked antihypertensive
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)