W355690
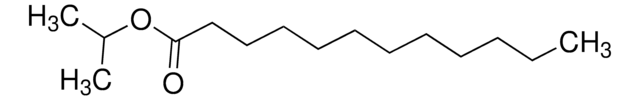
Isopropyl myristate
≥98%
Synonym(s):
Isopropyl tetradecanoate, Myristic acid isopropyl ester
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
Assay
≥98%
refractive index
n20/D 1.434 (lit.)
bp
193 °C/20 mmHg (lit.)
mp
~3 °C (lit.)
density
0.85 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
faint; fatty; oily
SMILES string
CCCCCCCCCCCCCC(=O)OC(C)C
InChI
1S/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-17(18)19-16(2)3/h16H,4-15H2,1-3H3
InChI key
AXISYYRBXTVTFY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Development of Nanostructured Lipid Carrier-Loaded Flavonoid-Enriched Zingiber officinale.: This research develops nanostructured lipid carriers for flavonoids derived from Zingiber officinale, employing Isopropyl myristate to stabilize these carriers and improve bioactive compound delivery (Shazwani SS et al., 2024).
- Comparative evaluation of physical and chemical enhancement techniques for transdermal delivery of linagliptin.: The study compares various enhancement methods for transdermal drug delivery, including the use of Isopropyl myristate, highlighting its effectiveness in permeation enhancement techniques (Karve T et al., 2024).
- Optimization of Conditions of Zanthoxylum Alkylamides Liposomes by Response Surface Methodology and the Absorption Characteristics of Liposomes in the Caco-2 Cell Monolayer Model.: This research optimizes liposome formulations containing Zanthoxylum alkylamides, with Isopropyl myristate being crucial in liposome stability and absorption characteristics (Wang R et al., 2024).
Biochem/physiol Actions
Disclaimer
Storage Class Code
10 - Combustible liquids
WGK
awg
Flash Point(F)
>302.0 °F
Flash Point(C)
> 150 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service