P38706
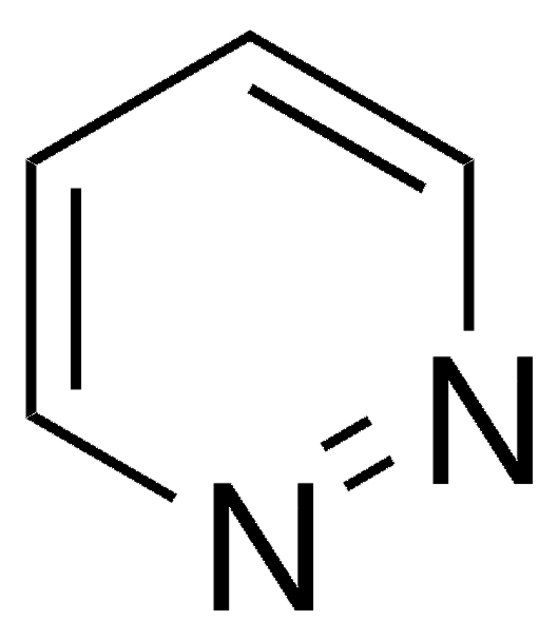
Phthalazine
98%
Synonym(s):
β-Phenodiazine, 2,3-Benzodiazine, 2,3-Diazanaphthalene, Benzo[d]pyridazine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H6N2
CAS Number:
Molecular Weight:
130.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
189 °C/29 mmHg (lit.)
mp
89-92 °C (lit.)
storage temp.
2-8°C
SMILES string
c1ccc2cnncc2c1
InChI
1S/C8H6N2/c1-2-4-8-6-10-9-5-7(8)3-1/h1-6H
InChI key
LFSXCDWNBUNEEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Muta. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michał Achmatowicz et al.
The Journal of organic chemistry, 74(2), 795-809 (2008-12-18)
p38 MAP kinase inhibitors have attracted considerable interest as potential agents for the treatment of inflammatory diseases. Herein, we describe a concise and efficient synthesis of inhibitor 1 that is based on a phthalazine scaffold. Highlights of our approach include
Manuel Sánchez-Moreno et al.
The Journal of antimicrobial chemotherapy, 67(2), 387-397 (2011-12-01)
To evaluate the in vitro leishmanicidal activity of imidazole-based (1-4) and pyrazole-based (5-6) benzo[g]phthalazine derivatives against Leishmania infantum and Leishmania braziliensis. The in vitro activity of compounds 1-6 was assayed on extracellular promastigote and axenic amastigote forms, and on intracellular
Fadi M Awadallah et al.
European journal of medicinal chemistry, 52, 14-21 (2012-03-24)
New phthalazine-based vasodilators were synthesized through the chloroacylation of the starting compound 1-hydrazinophthalazine 4 to give the two key intermediates 5 and 7. These intermediates were used to alkylate various cyclic amines to furnish the final compounds 6a-h and 8a-h.
Mehdi Rashidi et al.
Inorganic chemistry, 49(18), 8435-8443 (2010-08-18)
The reaction of phthalazine with the binuclear organoplatinum complexes [Me(2)Pt(μ-SMe(2))(μ-dppm)PtR(2)], R = Me, Ph, 4-tolyl or R(2) = (CH(2))(4), dppm = bis(diphenylphosphino)methane, gives the corresponding complexes [Me(2)Pt(μ-phthalazine)(μ-dppm)PtR(2)] by displacement of the bridging dimethylsulfide ligand. The structures of [Me(2)Pt(μ-SMe(2))(μ-dppm)PtMe(2)] and [Me(2)Pt(μ-phthalazine)(μ-dppm)PtMe(2)]
Hyungchul Kim et al.
International journal of pharmaceutics, 377(1-2), 105-111 (2009-05-26)
Investigation of the use of solution NMR spectroscopy to determine the effect of organic solvents on chemical shift changes in bases on addition of acids is reported. This information can be useful in the evaluation of solvents and counterion selection
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service