All Photos(1)
About This Item
Linear Formula:
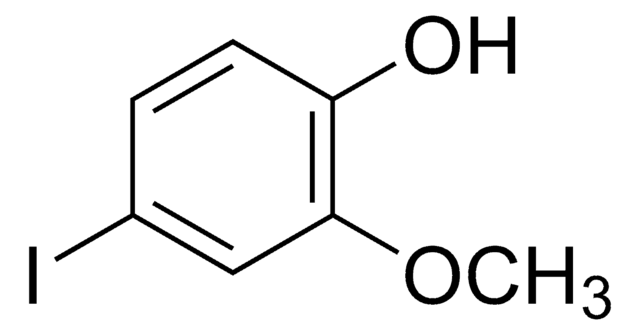
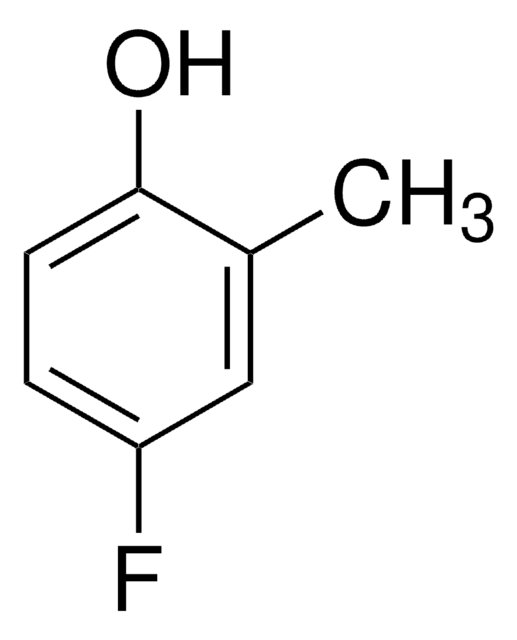
FC6H3(OCH3)OH
CAS Number:
Molecular Weight:
142.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.517 (lit.)
bp
195 °C (lit.)
density
1.247 g/mL at 25 °C (lit.)
SMILES string
COc1cc(F)ccc1O
InChI
1S/C7H7FO2/c1-10-7-4-5(8)2-3-6(7)9/h2-4,9H,1H3
InChI key
OULGLTLTWBZBLO-UHFFFAOYSA-N
General description
4-Fluoro-2-methoxyphenol is a fluorinated methoxy-substituted catechol analog.
Application
4-Fluoro-2-methoxyphenol may be used in the synthesis of:
- 4-halo-masked o-benzoquinones (MOBs)
- fluorinated masked o-benzoquinone
- poly(4-fluoro-2-methoxyphenol)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
214.9 °F - closed cup
Flash Point(C)
101.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A fluorinated masked o-benzoquinone.
Patrick TB, et al.
Journal of Fluorine Chemistry, 125(12), 1965-1966 (2007)
Novel photoconductive polyfluorophenol synthesized by an enzyme.
Zaragoza-Gasca P, et al.
Journal of Molecular Catalysis. B, Enzymatic, 72(1), 25-27 (2011)
Ana Carolina de Almeida et al.
European journal of pharmacology, 660(2-3), 445-453 (2011-04-19)
Apocynin, a methoxy-substituted catechol (4-hydroxy-3-methoxyacetophenone), originally extracted from the roots of Picrorhiza kurroa, has been extensively used as a non-toxic inhibitor of the multienzymatic complex NADPH oxidase. We discovered that the analogous methoxy-substituted catechol, 4-Fluoro-2-methoxyphenol (F-apocynin), in which the acetyl
Diels-Alder reactions of halogenated masked o-benzoquinones: synthesis of halogen-substituted bicyclo [2.2. 2] octenones.
Tetrahedron Letters, 50(7), 773-775 (2009)
P K Chakraborty et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 42(7), 673-681 (1991-01-01)
The synthesis of 4-[18F]fluoroguaiacol (4-[18F]fluoro-2-methoxyphenol) has been achieved in no-carrier-added form starting from 2-methoxy-4-nitrobenzaldehyde, using nucleophilic aromatic substitution by [18F]fluoride followed by Baeyer-Villiger oxidation of the benzaldehyde to the phenol. Demethylation with boron tribromide gave 4-[18F]fluorocatechol (1,2-dihydroxy-4-[18F]fluorobenzene) with an overall
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service