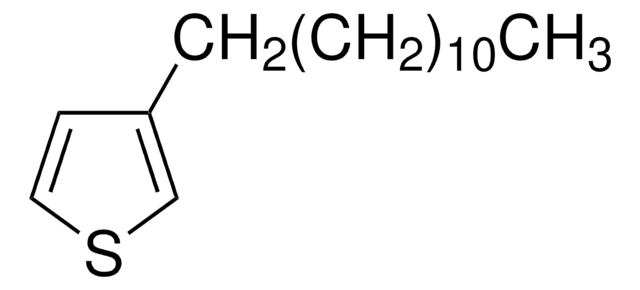456373
2,5-Dibromo-3-hexylthiophene
97%
Synonym(s):
2,5-Dibromo-3-hex-1-ylthiophene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H14Br2S
CAS Number:
Molecular Weight:
326.09
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.557 (lit.)
density
1.521 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCCCc1cc(Br)sc1Br
InChI
1S/C10H14Br2S/c1-2-3-4-5-6-8-7-9(11)13-10(8)12/h7H,2-6H2,1H3
InChI key
NSYFIAVPXHGRSH-UHFFFAOYSA-N
General description
2,5-Dibromo-3-hexylthiophene is a 2,5 coupled conductive polymer with conjugated polythiophene based system, which has a controllable band gap.
Application
Conducting polymer precursor.
It can be used as a monomer with 5,5′-dibromo-3,3′-dihexyl-2,2′-bithiophene, which can synthesize regioregular-P3HT-regiosymmetric-P3HT (a diblock polymer) for organic electronics based applications. It can also be used in the synthesis of P3HT, which can be potentially used for photocatalytic applications.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charge Transport in Conjugated Polymers with Pendent Stable Radical Groups.
Zhang Y, et al.
Chemistry of Materials (2018)
Paulina Powroznik et al.
Sensors (Basel, Switzerland), 20(2) (2020-01-19)
In the present work, we report the use of regioregular poly(3-hexyltiophene) polymer (RR-P3HT) as a potential light-activated material for sensing the chemical nerve agent simulant dimethyl methylphosphonate (DMMP). The electrical response of thick films of RR-P3HT, deposited by spray-coating method
Evidence of Ni (0) complex diffusion during grignard metathesis polymerization of 2, 5-dibromo-3-hexylthiophene.
Achord BC and Rawlins JW
Macromolecules, 42(22), 8634-8639 (2009)
Uniform electroactive fibre-like micelle nanowires for organic electronics.
Li X, et al.
Nature Communications, 8(22), 15909-15909 (2017)
Xiaoyu Li et al.
Nature communications, 8, 15909-15909 (2017-06-27)
Micelles formed by the self-assembly of block copolymers in selective solvents have attracted widespread attention and have uses in a wide variety of fields, whereas applications based on their electronic properties are virtually unexplored. Herein we describe studies of solution-processable
Articles
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)