28910
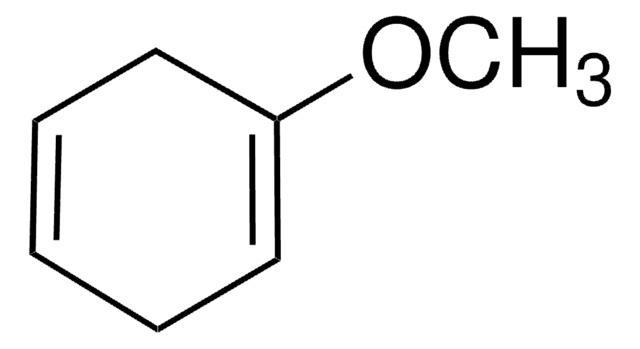
1,4-Cyclohexadiene
purum, ≥97.0% (GC)
Synonym(s):
1,4-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
1900733
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (GC)
form
liquid
contains
~0.2% 2,6-di-tert-butyl-4-methylphenol as stabilizer
refractive index
n20/D 1.472 (lit.)
n20/D 1.473
bp
88-89 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
SMILES string
C1C=CCC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-2,5-6H,3-4H2
InChI key
UVJHQYIOXKWHFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,4-Cyclohexadiene is an effective hydrogen donor for catalytic hydrogenation reaction. The interaction between graphene segments and 1,4-cyclohexadiene was stuided using density-functional tight-binding (DFTB) method.
Application
1,4-Cyclohexadiene (1,4-CHD) was used to study the formation of parent ion from heavy fragmentation of 1,4-CHD on irradiation with a high-intensity laser pulse.
Other Notes
Reagent for selectively cleaving benzyl esters in the presence of benzyl ethers by catalytic H-transfer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1A - Flam. Liq. 2 - Muta. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A.M. Felix et al.
The Journal of Organic Chemistry, 43, 4194-4194 (1978)
A key factor in parent and fragment ion formation on irradiation with an intense femtosecond laser pulse.
Chemical Physics Letters, 563-570 null
Rapid removal of protecting groups from peptides by catalytic transfer hydrogenation with 1, 4-cyclohexadiene.
The Journal of Organic Chemistry, 43(21), 4194-4196 (1978)
Kazutada Ikeuchi et al.
Organic letters, 14(23), 6016-6019 (2012-11-15)
Asymmetric bromolactonization of prochiral cyclohexadiene derivatives with N-bromosuccimide proceeded in the presence of (DHQD)(2)PHAL as a chiral catalyst to afford the corresponding bromolactones with up to 93% ee. This reaction was also applicable to the kinetic resolution of a racemic
Kyung-Bin Cho et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(33), 10444-10453 (2012-06-21)
The experimentally measured bimolecular reaction rate constant, k(2), should in principle correlate with the theoretically calculated rate-limiting free energy barrier, ΔG(≠), through the Eyring equation, but it fails quite often to do so due to the inability of current computational
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service