T7951
Tau Protein Ladder, 6 isoforms human
recombinant, expressed in E. coli, ≥90% (SDS-PAGE), buffered aqueous glycerol solution
Synonym(s):
Tau Protein Ladder
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in E. coli
Assay
≥90% (SDS-PAGE)
form
buffered aqueous glycerol solution
mol wt
36800
39700
40000
42600
42900
45900
composition
dodecyl sulphate sodium salt, 1-5%
glycerine, 20-30%
mercaptoethanol, 10-20%
technique(s)
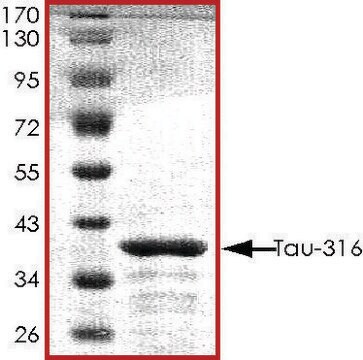
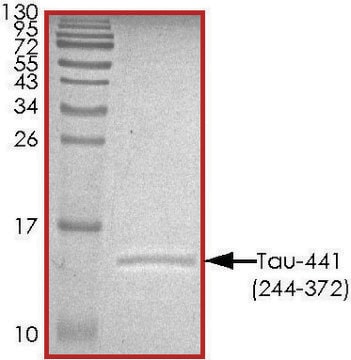
immunoelectrophoresis: 10-20 μL using recombinant Tau protein marker
western blot: 2-5 μL using recombinant Tau protein marker
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... MAPT(4137)
General description
Tau gene, spanning 100 kb with 16 exons, is mapped to human chromosome 17q21. The six alternative splice variants of protein ranging in size from 352-441 amino acids have been identified in human adult brain. Tau is a member of the microtubule-associated protein (MAP) family. It is predominantly expressed in neurons.
Application
Biochem/physiol Actions
Packaging
Specifications
Physical form
Storage and Stability
to qualified or authorized persons
antibody
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Target Organs
Liver,Heart
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service