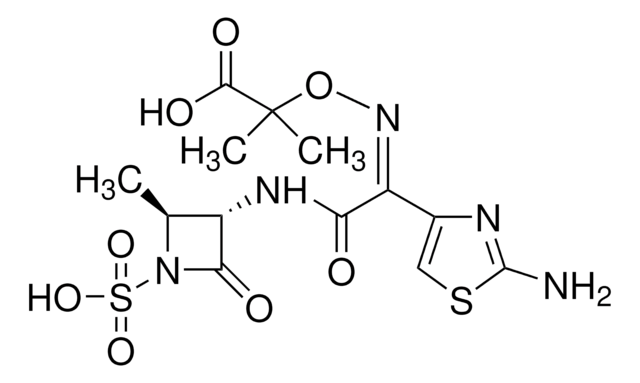
B0184
Bacteriorhodopsin from Halobacterium salinarum
native sequence, lyophilized powder
Synonym(s):
BR from H. salinarum, Bacterioopsin, Bacteriorhodopsin from Halobacterium halobium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Halobacterium salinarium
form
lyophilized powder
technique(s)
ligand binding assay: suitable
mass spectrometry (MS): suitable
UniProt accession no.
storage temp.
2-8°C
Gene Information
Halobacterium salinarium ... OE_RS05715(5953595) , VNG_RS05715(144807)
Looking for similar products? Visit Product Comparison Guide
General description
Bacteriorhodopsin (BR) is a covalent complex comprising bacterioopsin protein and retinal cofactor in the equimolar ratio. It corresponds to the molecular weight of 27kDa. BR belongs to the retinylidene class of proteins. It is a seven-membrane helical protein that acts as a photon-driven pump. BR can be used in studies of the folding and kenetics of β-helical proteins.
Bacteriorhodopsin is the prototypical "seven-helix" transmembrane protein (with seven α-helical domains), whose study led to advances in understanding G protein-coupled receptors (GPCRs). In Halobacteria, it acts as a light-harvesting protein, producing a proton gradient across the cell wall that is then used to drive biosynthetic processes.
Application
Bacteriorhodopsin from Halobacterium salinarum has been used:
- in generation of droplet lipid bilayer
- as a standard in quadrupole time-of-flight (QTOF) mass spectroscopy (MS)
- in the generation of protein-detergent complex and micelles for dynamic light scattering studies
Bacteriorhodopsin is of interest in the development of artificial retinas, optical associative processors, and three-dimensional memory storage devices.
Biochem/physiol Actions
A transmembrane retinylidine protein that functions as a proton pump driven by light energy in Holobacterium.
Bacteriorhodopsin (BR) from Halobacterium salinarum acts as a proton-driven pump. BR can be used in studies of the folding and kinetics of α-helical proteins. It is thermally stable and exhibits high photoelectric and photochemical efficiency. BR exists as trimer in a hexagonal lattice. Its photocycle intermediates are exploited in bioelectronics majorly in photoelectric and photochemical applications.
Preparation Note
Aqueous suspensions may be sonicated to achieve the desired homogeneity and may be stored for a short time at a temperature of 2-8 °C or at a temperature of -20 °C without time limitation.
Wild-type bacteriorhodopsin is isolated from Halobacterium salinarum strain S9 as purple membranes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jonathan P Schlebach et al.
Protein science : a publication of the Protein Society, 21(1), 97-106 (2011-11-19)
The elucidation of the physical principles that govern the folding and stability of membrane proteins is one of the greatest challenges in protein science. Several insights into the folding of α-helical membrane proteins have come from the investigation of the
High production of bacteriorhodopsin from wild type Halobacterium salinarum
Seyedkarimi MS, et al.
Extremophiles : Life Under Extreme Conditions, 19(5), 1021-1028 (2015)
A review on bacteriorhodopsin-based bioelectronic devices
Li YT, et al.
Sensors, 18(5), 1368-1368 (2018)
Light-Patterned Current Generation in a Droplet Bilayer Array
Schild V, et al.
Scientific reports, 7, 46585-46585 (2017)
Best practices and benchmarks for intact protein analysis for top-down mass spectrometry
Donnelly DP, et al,
Nature Methods, 16(7), 587-594 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service