75580
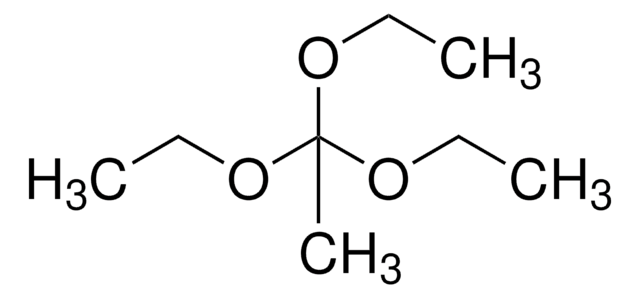
Triethyl orthoacetate
purum, ≥98.0% (GC)
Synonym(s):
1,1,1-Triethoxyethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(OC2H5)3
CAS Number:
Molecular Weight:
162.23
Beilstein:
506201
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.396 (lit.)
n20/D 1.396
bp
142 °C (lit.)
density
0.885 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CCOC(C)(OCC)OCC
InChI
1S/C8H18O3/c1-5-9-8(4,10-6-2)11-7-3/h5-7H2,1-4H3
InChI key
NDQXKKFRNOPRDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Triethyl orthoacetate is a general reagent used to functionalize alcohols with acetate groups. It can be used in following reactions:
- Stereocontrolled total synthesis of a naturally occuring indole alkaloid, (−)-aspidophytine.
- Conversion of allylic alcohols to γ,δ-unsaturated esters under mild acidic condition, a reaction popularly known as Johnson–Claisen rearrangement.
- Synthesis of heterocycles such as 2-oxazolines and quinazolin-4(3H)-one derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
102.2 °F - Non-equilibrium method
Flash Point(C)
39 °C - Non-equilibrium method
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Clay catalysis: condensation of orthoesters with O-substituted aminoaromatics into heterocycles.
Villemin D, et al.
Synthetic Communications, 26(15), 2895-2899 (1996)
An efficient and versatile method for the synthesis of optically active 2-oxazolines: an acid-catalyzed condensation of ortho esters with amino alcohols.
Kamata K, et al.
The Journal of Organic Chemistry, 63(9), 113-3116 (1998)
A new approach to the facile synthesis of mono-and disubstituted quinazolin-4 (3H)-ones under solvent-free conditions.
Salehi P, et al.
Tetrahedron Letters, 46(41), 7051-7053 (2005)
Stereocontrolled total synthesis of (−)-aspidophytine.
Sumi S, et al.
Tetrahedron, 59(43), 8571-8587 (2003)
Reaction of orthoesters with alcohols in the presence of acidic catalysts: A study.
Kumar, HM et al.
Indian J. Chem. B, 44B(8), 1686-1692 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service