H57009
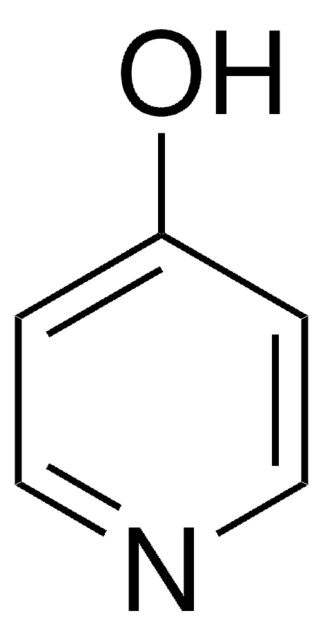
3-Hydroxypyridine
98%
Synonym(s):
3-Pyridinol, 3-Pyridone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105699
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
125-128 °C (lit.)
SMILES string
Oc1cccnc1
InChI
1S/C5H5NO/c7-5-2-1-3-6-4-5/h1-4,7H
InChI key
GRFNBEZIAWKNCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fazlul Huq et al.
European journal of medicinal chemistry, 39(8), 691-697 (2004-07-28)
Four trans-planaramineplatinum(II) complexes code named YH9, YH10, YH11 and YH12, each of the form trans-PtL(NH(3))Cl(2) where L = 2-hydroxypyridine and 3-hydroxypyridine, imidazole, and imidazo(1,2-alpha)pyridine for YH9, YH10, YH11 and YH12, respectively. All of the compounds have significant anticancer activity against
G S M Kiruba et al.
The Journal of organic chemistry, 68(7), 2874-2881 (2003-03-29)
The tautomeric equilibria of a series of 3-hydroxypyridine derivatives including pyridoxal-5'-phosphate (PLP), the active form of vitamin B(6), have been studied using density functional calculations (B3LYP/6-311+G//B3LYP/6-31G) in the gas phase and in different solvents. Three different approaches, namely continuum, discrete
Raffaele Saladino et al.
Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life, 41(4), 317-330 (2011-03-23)
The thermal condensation of formamide in the presence of mineral borates is reported. The products afforded are precursors of nucleic acids, amino acids derivatives and carboxylic acids. The efficiency and the selectivity of the reaction was studied in relation to
N L Sakina et al.
Bulletin of experimental biology and medicine, 147(2), 193-195 (2009-06-11)
Photoprotective activity of heteroaromatic compounds (derivatives of 3-hydroxypyridine, amino-6-hydroxybenzothiazole, and 5-hydroxybenzimidazole) was studied in the system of UV-induced cardiolipin peroxidation. Although all three compounds had the antioxidant effect during free radical oxidation of luminol, only derivatives of amino-6-hydroxybenzothiazole and 5-hydroxybenzimidazole
E B Watkins et al.
Bioorganic & medicinal chemistry letters, 11(16), 2099-2100 (2001-08-22)
Tyrosine phenol-lyase from Citrobacter freundii synthesizes 2-aza-L-tyrosine and 3-aza-L-tyrosine from 3-hydroxypyridine and 2-hydroxypyridine, respectively, and ammonium pyruvate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service