852473
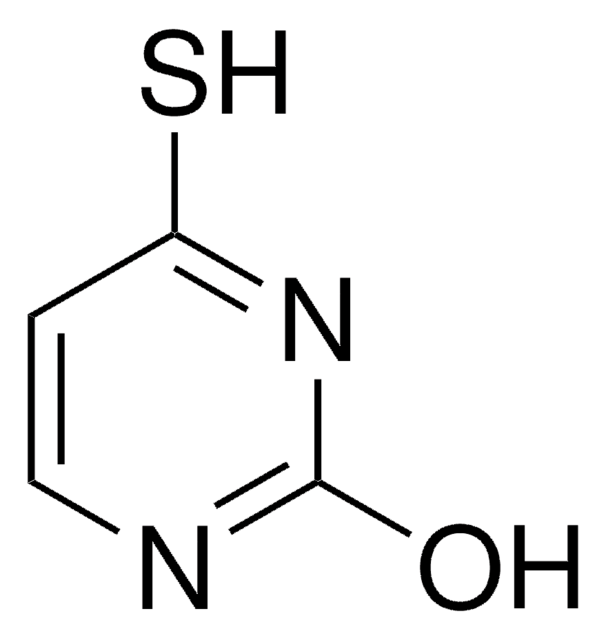
5-Bromouracil
98%
Synonym(s):
5-Bromo-2,4-dihydroxypyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H3BrN2O2
CAS Number:
Molecular Weight:
190.98
Beilstein:
127176
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
>300 °C (lit.)
functional group
bromo
SMILES string
BrC1=CNC(=O)NC1=O
InChI
1S/C4H3BrN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
InChI key
LQLQRFGHAALLLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zejun Li et al.
The journal of physical chemistry. B, 115(46), 13668-13673 (2011-09-10)
The reaction of low-energy electrons (LEEs; 10 eV) with 5'-TpXpT-3' (TXT), where X is uracil (U), thymine (T), and 5-bromouracil (5BrU), was examined by HPLC-UV analysis. The presence of 5BrU increased total damage by >50%. The radiation products of T5BrUT
E Itälä et al.
The Journal of chemical physics, 133(15), 154316-154316 (2010-10-26)
Photofragmentation of thymine and 5-bromouracil into cation and neutral fragments following the core ionization by soft x-rays using photoelectron-photoion-photoion coincidence technique has been studied. The fragment ion mass spectra were recorded in coincidence with the C 1s photoelectron spectra. In
Pierre Daublain et al.
The journal of physical chemistry. B, 114(45), 14265-14272 (2010-02-04)
The photophysical and photochemical behavior of a series of hairpin-forming DNA conjugates possessing a 5'-tethered pyrenecarboxamide chromophore and one or two bromouracil bases has been investigated. Quenching of the pyrene fluorescence and transient absorption spectra characteristic of the pyrene cation
Ryu Tashiro et al.
Journal of the American Chemical Society, 132(41), 14361-14363 (2010-09-30)
We have investigated the products of (Br)U in excess electron transfer and have demonstrated that in DNA the proportion of products changes with the distance between the donor and acceptor. On the basis of a labeling experiment using H(2)(18)O, we
Hironobu Morinaga et al.
Bioorganic & medicinal chemistry, 21(2), 466-469 (2012-12-26)
5-Bromouracil ((Br)U) was incorporated into three types of synthetic RNA and the products of the photoirradiated (Br)U-containing RNAs were investigated using HPLC and MS analysis. The photoirradiation of r(GCA(Br)UGC)(2) and r(CGAA(Br)UUGC)/r(GCAAUUCG) in A-form RNA produced the corresponding 2'-keto adenosine ((keto)A)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service