632961
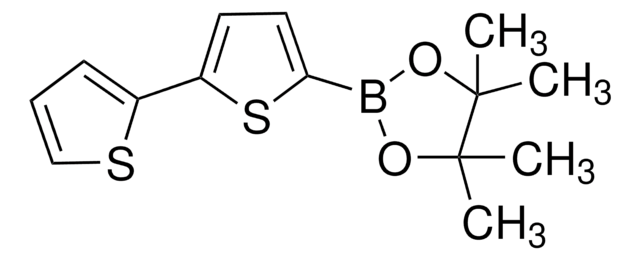
5′-Hexyl-2,2′-bithiophene-5-boronic acid pinacol ester
97%
Synonym(s):
2-(5′-Hexyl-2,2′-bithien-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-[5′-hexyl-2,2′-bithien-5-yl]-1,3,2-dioxaborolane, 5′-N-Hexyl-2,2′-bithiophene-5-boronic acid pinacol ester, 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-5′-N-hexyl-2,2′-bithiophene, 5-Hexyl-5′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,2′-bithiophene
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
mp
36-40 °C (lit.)
SMILES string
CCCCCCc1ccc(s1)-c2ccc(s2)B3OC(C)(C)C(C)(C)O3
InChI
1S/C20H29BO2S2/c1-6-7-8-9-10-15-11-12-16(24-15)17-13-14-18(25-17)21-22-19(2,3)20(4,5)23-21/h11-14H,6-10H2,1-5H3
InChI key
XTTRNSNHDCYSEL-UHFFFAOYSA-N
Related Categories
Application
- Suzuki-Miyaura cross-coupling reactions and shape-shifting in contorted dibenzotetrathienocoronenes
- Oligothiophene self-assembly induction into fibers with tunable shape and function
- Stille coupling and p-conjugated packing structure and hole mobility of bithiophene-bithiazole copolymers with alkyl-thiophene side chains
Reagent used in Preparation of
- Solution-processed ambipolar field-effect transistor
- Light harvesting small molecules for use in solution-processed small molecule bulk heterojunction solar cell devices
- Light-emitting diode (OLED) materials
- Unsymmetric substituted benzothiadiazole-containing vinyl monomers for RAFT polymerization
- Pd-catalyzed condensations and synthesis of isoindigo-based oligothiophenes for molecuar bulk heterojunction solar cells
- Thiophene-benzothiadiazole based donor-acceptor-donor materials
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service