All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O3
CAS Number:
Molecular Weight:
128.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
59-62 °C (lit.)
SMILES string
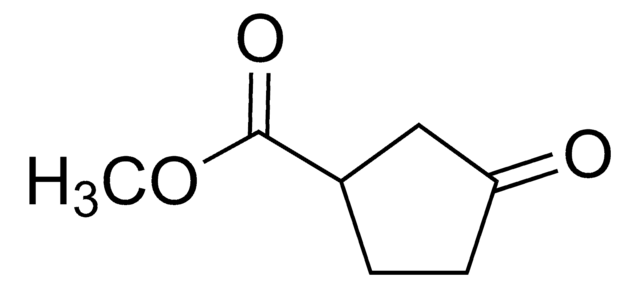
OC(=O)C1CCC(=O)C1
InChI
1S/C6H8O3/c7-5-2-1-4(3-5)6(8)9/h4H,1-3H2,(H,8,9)
InChI key
RDSNBKRWKBMPOP-UHFFFAOYSA-N
Related Categories
General description
3-Oxo-1-cyclopentanecarboxylic acid , also known as 3-oxocyclopentanecarboxylic acid, is a keto acid derivative. It undergoes Curtius rearrangement with diphenyl phosphoryl azide and triethylamine in tert-butanol to form the corresponding boc-protected 1-(3-oxo)urea derivative.
Application
3-Oxo-1-cyclopentanecarboxylic acid may be used in the preparation of 3-hydroxycyclopentanecarboxylic acid via hydrogenation.
Substrate used in a study of biohydroxylation with mutants of cytochrome P450 BM-3.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Boc-protected 1-(3-oxocycloalkyl) ureas via a one-step Curtius rearrangement: mechanism and scope.
Sun X, et al.
Tetrahedron Letters, 55(4), 842-844 (2014)
Dieter F Münzer et al.
Chemical communications (Cambridge, England), (20), 2597-2599 (2005-05-19)
Substrate engineered, achiral carboxylic acid derivative was biohydroxylated with various mutants of cytochrome P450 BM-3 to give two out of the four possible diastereoisomers in high de and ee. The BM-3 mutants exhibit up to 9200 total turnovers for hydroxylation
Studies of Configuration. V. The Preparation and Configuration of cis-3-Methoxycyclopentanecarboxylic Acid.
Noyce D and Fessenden J.
The Journal of Organic Chemistry, 24(5), 715-717 (1959)
Articles
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service