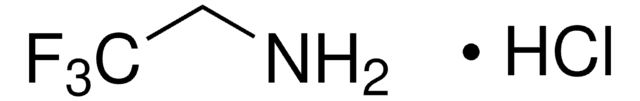
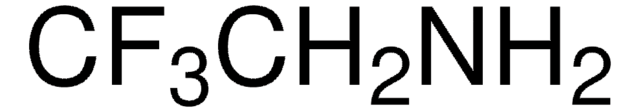429058
2-Fluoroethylamine hydrochloride
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
FCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
99.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
solid
mp
99-103 °C (lit.)
SMILES string
Cl.NCCF
InChI
1S/C2H6FN.ClH/c3-1-2-4;/h1-2,4H2;1H
InChI key
YRRZGBOZBIVMJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Fluoroethylamine hydrochloride is a fluorinated alkylammonium salt. Studies suggest that adding a fluorine atom to it leads to double gauche effect in the resulting 2,2-difluoroethylamine chloride. Microwave spectral studies for 2-fluoroethylamine has been conducted.
Application
2-Fluoroethylamine hydrochloride may be used in the synthesis of the following:
- 2-fluoroethylisocyanate
- 1,3-bis-(2-fluoroethyl) urea (BFU)
- N-2-fluoroethylmaleimide
- [Cu4(O)(Ln)2(CH3COO)4] where HL= 4-methyl-2,6-bis(2-fluoroethyliminomethyl) phenol
- 1-O-(4-(2-fluoroethyl-carbamoyloxymethyl)-2-nitrophenyl)-O-β-D-glucopyronuronate (FEAnGA)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetranuclear copper (II)-Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene.
Roy P and Manassero M.
Dalton Transactions, 39(6), 1539-1545 (2010)
Microwave-spectrum, conformational equilibrium, intramolecular hydrogen-bonding, dipole-moments, N-14 nuclear-quadrupole coupling-constants and centrifugal-distortion constants of 2-fluoroethylamine.
Marstokk KM and Mollendal H.
Acta Chemica Scandinavica, 34(1), 15-29 (1980)
Synthesis of 18F-labelled 2-fluoroethyl-nitrosoureas.
Farrokhzad S, et al.
Canadian Journal of Chemistry, 62(11), 2107-2112 (1984)
Inês F Antunes et al.
Bioconjugate chemistry, 21(5), 911-920 (2010-04-27)
To increase the therapeutic index of chemotherapeutic drugs, prodrugs have been investigated as anticancer agents, as they may present fewer cytotoxic side effects than conventional cytotoxic drugs, while therapeutic efficacy is maintained or even increased. Extracellular beta-glucuronidase (beta-GUS) in the
Development of a new thiol site-specific prosthetic group and its conjugation with [Cys40]-exendin-4 for in vivo targeting of insulinomas.
Yue X, et al.
Bioconjugate Chemistry, 24(7), 1191-1200 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service