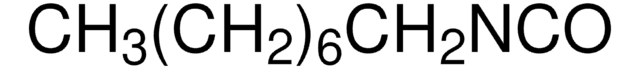All Photos(1)
About This Item
Linear Formula:
CH3(CH2)5NCO
CAS Number:
Molecular Weight:
127.18
Beilstein:
1751836
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
162-164 °C (lit.)
density
0.873 g/mL at 25 °C (lit.)
functional group
amine
isocyanate
SMILES string
CCCCCCN=C=O
InChI
1S/C7H13NO/c1-2-3-4-5-6-8-7-9/h2-6H2,1H3
InChI key
ANJPRQPHZGHVQB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hexyl isocyanate has been investigated for its antigenicity in the guinea pig animal model of hapten-specific respiratory hypersensitivity. Polymerization of n-hexyl isocyanate using rare earth tris(2,6-di-tert-butyl-4-methylphenolate) [Ln(OAr)3] as initiator has been studied.
Application
Hexyl isocyanate may be employed for the synthesis of following:
- rigid-rod, helical isocyanate-based macromonomers
- rod-coil-rod triblock copolymers with 2-vinylpyridine, by living anionic polymerization
- chiral poly(n-hexyl isocyanate) (PHIC) macromonomers, by living anionic polymerization
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pulmonary hypersensitivity to hexyl isocyanate-ovalbumin aerosol in guinea pigs.
M H Karol et al.
Toxicology and applied pharmacology, 51(1), 73-80 (1979-10-01)
Boer Liu et al.
Molecules (Basel, Switzerland), 26(15) (2021-08-08)
This work reveals the influence of pendant hydrogen bonding strength and distribution on self-assembly and the resulting thermomechanical properties of A-AB-A triblock copolymers. Reversible addition-fragmentation chain transfer polymerization afforded a library of A-AB-A acrylic triblock copolymers, wherein the A unit
Synthesis and Self-Assembly Studies of Amphiphilic Poly (n-hexyl isocyanate)-b lock-poly (2-vinylpyridine)-b lock-poly (n-hexyl isocyanate) Rod-Coil-Rod Triblock Copolymer.
Rahman M, et al.
Macromolecules, 39(15), 5009-5014 (2006)
Chiroptical Properties of Graft Copolymers Containing Chiral Poly (n-hexyl isocyanate) as a Side Chain.
Shah PN, et al.
Macromolecules, 44(20), 7917-7925 (2011)
Maresa Sonnabend et al.
Polymers, 13(12) (2021-07-03)
Due to reasons of sustainability and conservation of resources, polyurethane (PU)-based systems with preferably neutral carbon footprints are in increased focus of research and development. The proper design and development of bio-based polyols are of particular interest since such polyols
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service