All Photos(1)
About This Item
Empirical Formula (Hill Notation):
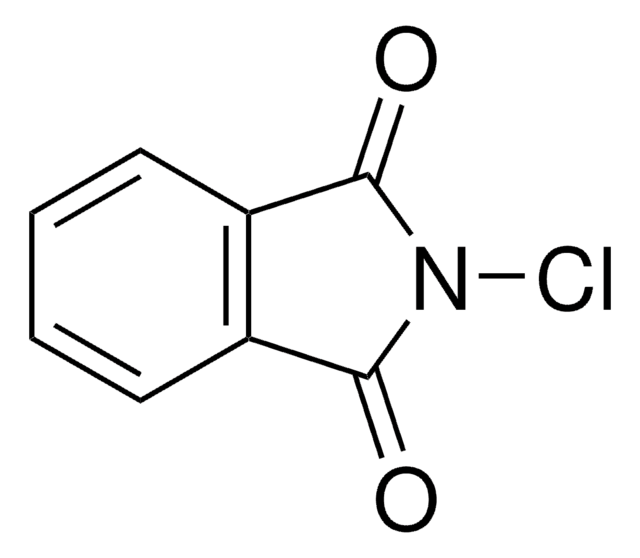
C8H4BrNO2
CAS Number:
Molecular Weight:
226.03
Beilstein:
131211
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
194-198 °C (lit.)
SMILES string
BrN1C(=O)c2ccccc2C1=O
InChI
1S/C8H4BrNO2/c9-10-7(11)5-3-1-2-4-6(5)8(10)12/h1-4H
InChI key
MARXMDRWROUXMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Bromophthalimide has been used:
- as reagent in allylic amination reactions of alkenes
- brominating reagent in enantioselective synthesis of multisubstituted biaryl derivatives by chiral phosphoric acid catalyzed asymmetric bromination
- as a titrant in titrimetric determination of isoniazid in pure form or in tablets
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Titrimetric determination of para-aminobenzoic acid using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
Journal of pharmaceutical and biomedical analysis, 7(5), 627-631 (1989-01-01)
Feng Chen et al.
Journal of the American Chemical Society, 135(4), 1232-1235 (2013-01-15)
A catalytic asymmetric bromocyclization of trisubstituted olefinic amides that uses a C(2)-symmetric mannitol-derived cyclic selenium catalyst and a stoichiometric amount of N-bromophthalimide is reported. The resulting enantioenriched pyrrolidine products, which contain two stereogenic centers, can undergo rearrangement to yield 2,3-disubstituted
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 62(4-5), 918-925 (2005-06-14)
Fourier transform infrared (FT-IR) spectra of phthalimide and N-bromophthalimide have been recorded in the range of 4000-400 cm-1. With the hope of providing more and effective information on the fundamental vibrations, a normal coordinate analysis has been performed on phthalimide
Ying Wei et al.
Organic letters, 15(20), 5186-5189 (2013-10-10)
Allylic amination reactions of alkenes, with an NBP (N-bromophthalimide) or NBS (N-bromosuccinimide)/DBU combination, were developed, in which both internal and external nitrogen nucleophiles can be installed directly. Dual activation of NBS or NBP by DBU leads to more electrophilic bromine
Titrimetric methods for the determination of some sulpha drugs using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
The Analyst, 113(9), 1369-1372 (1988-09-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service