S0417
Silibinin
≥98% (HPLC)
Synonym(s):
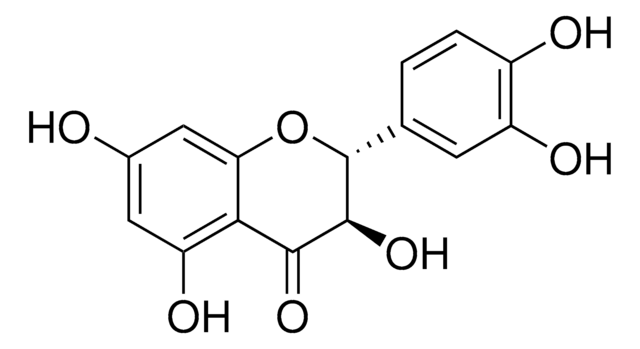
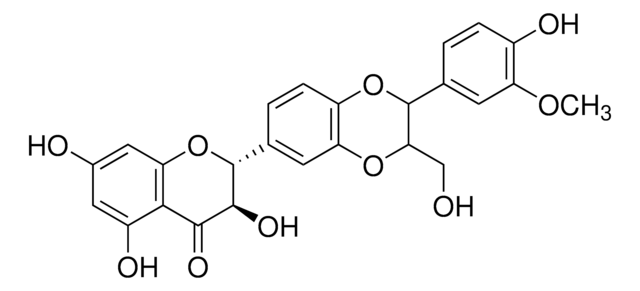
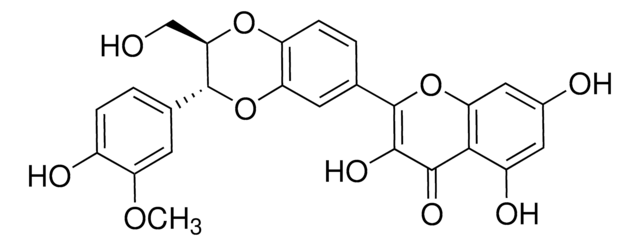
2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-6-(3,5,7-trihydroxy-4-oxobenzopyran-2-yl)benzodioxin, Silybin
About This Item
Recommended Products
Assay
≥98% (HPLC)
storage temp.
−20°C
SMILES string
COc1cc(ccc1O)[C@@H]2Oc3cc(ccc3O[C@H]2CO)[C@H]4Oc5cc(O)cc(O)c5C(=O)[C@H]4O
InChI
1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23+,24-,25?/m0/s1
InChI key
SEBFKMXJBCUCAI-ILJKEPSESA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effect on gene expression levels of various proteins involved in chromatin regulations of prostate cancer, by real time polymerase chain reaction (RT-PCR)
- to study its effect on cell proliferation in platelet-derived growth factor (PDGF)-treated human tenon′s fibroblasts (HTFs) by investigating the expression of proliferating cell nuclear antigen (PCNA) and by water-soluble tetrazolium salt (WST-1) assay
- to examine its effect on gene expression levels of stromelysine 1 (STM1), acetyl hexoseamines and collagen production during skin wound healing
- to study its inhibitory effect on Escherichia coli ATP synthase
Biochem/physiol Actions
Components
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Chronic inflammation is an underlying factor in the development and progression of many of the chronic diseases of aging, such as arthritis, atherosclerosis, diabetes, and cancer.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service