All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H38N6O4
CAS Number:
Molecular Weight:
402.53
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Lys-Lys-Lys, ≥97% (TLC)
Assay
≥97% (TLC)
form
powder
composition
Peptide content, ~60%
color
white to off-white
storage temp.
−20°C
SMILES string
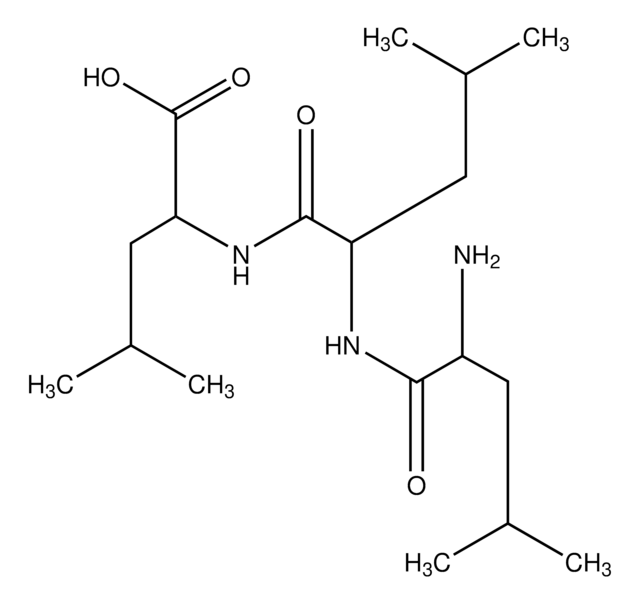
NCCCCC(N)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(O)=O
InChI
1S/C18H38N6O4/c19-10-4-1-7-13(22)16(25)23-14(8-2-5-11-20)17(26)24-15(18(27)28)9-3-6-12-21/h13-15H,1-12,19-22H2,(H,23,25)(H,24,26)(H,27,28)
InChI key
WBSCNDJQPKSPII-UHFFFAOYSA-N
Amino Acid Sequence
Lys-Lys-Lys
Application
Short poly-L-lysines polypeptides such as trilysine (tri-L-lysine, lys3); tetralysine (tetra-L-lysine, lys4) and pentalysine (penta-L-lysine, lys5) are cationic moieties that may be used in the construction of gene delivery vectors and DNA nanoparticles.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aboli A Rane et al.
PloS one, 6(6), e21571-e21571 (2011-07-07)
Several injectable materials have been shown to preserve or improve cardiac function as well as prevent or slow left ventricular (LV) remodeling post-myocardial infarction (MI). However, it is unclear as to whether it is the structural support or the bioactivity
Sandrine Perrier et al.
Journal of the American Chemical Society, 128(17), 5703-5710 (2006-04-28)
Formation of DNA-protein cross-links involving the initial formation of a guanine radical cation was investigated. For this purpose, riboflavin-mediated photosensitization of a TGT oligonucleotide in aerated aqueous solution in the presence of the KKK tripeptide was performed. We have shown
Peter G Millili et al.
Microscopy research and technique, 73(9), 866-877 (2010-03-17)
Polycationic polymers have been used to condense therapeutic DNA into submicron particles, offering protection from shear-induced or enzymatic degradation. However, the spontaneous nature of this self-assembly process gives rise to the formation of multimolecular aggregates, resulting in significant polyplex heterogeneity.
Erik Wernersson et al.
The journal of physical chemistry. B, 114(36), 11934-11941 (2010-08-24)
Domains rich in cationic amino acids are ubiquitous in peptides with the ability to cross cell membranes, which is likely related to the binding of such polypeptides to anionic groups on the membrane surface. To shed more light on these
Magdalena Stobiecka et al.
Biomaterials, 32(12), 3312-3321 (2011-02-11)
In view of the prospective applications of polyamine coatings in functional gold nanoparticles for use as carriers in gene delivery systems, in tissue repair and as bactericidal and virucidal non-toxic vehicle, we have investigated the interactions of poly-l-lysine (PLL) with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service