62335
Lipoprotein Lipase from Pseudomonas sp.
lyophilized, powder, ≥1200 U/mg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bacterial (Pseudomonas spp.)
Quality Level
form
powder
quality
lyophilized
specific activity
≥1200 U/mg
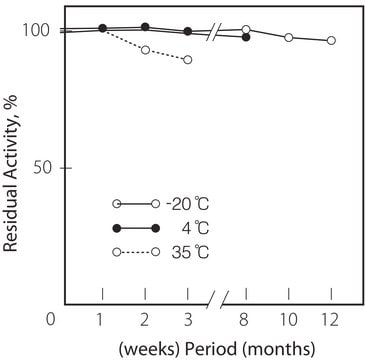
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Lipoprotein Lipase (LPL) is majorly secreted by myocytes and adipocytes in humans and is crucial for triglyceride homeostatis. Mutations in the catalytic domain of LPL impairs its interaction with glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1). The N-terminal catalytic domain is essential for lipolysis. The C-terminal is crucial for binding lipoproteins. Altered LPL levels may play role in the pathogenesis of atherosclerosis, coronary heart disease and chronic lymphocytic leukemia.
Application
Lipoprotein Lipase from Pseudomonas sp. has been used in the enzymatic erosion studies in gamma irradiated poly(trimethylene carbonate) (PTMC) films.
Biochem/physiol Actions
Malic dehydrogenase catalyzes the dehydrogenation of L-malate by NAD+.
Lipoprotein lipase belongs to the family of triglyceride lipases. It hydrolyses triglycerides in triglyceride-rich ApoB-containing lipoproteins.
Unit Definition
1 U corresponds to the amount of enzyme which liberates 1 μmol oleic acid per minute at pH 8.0 and 40°C (triolein, Cat. No. 62314 as substrate)
Other Notes
Preparation of aldol acceptors (R)- and (S)-3-azido-2-hydroxypropanal via lipase-catalyzed resolution of the racemic acetal precursor; Effect of enzyme form on its properties in toluene
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C.H. von der Osten et al.
Journal of the American Chemical Society, 111, 3924-3924 (1989)
Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1
Voss CV, et al.
Proceedings of the National Academy of Sciences of the USA, 108(19), 7980-7984 (2011)
A lipoprotein lipase (LPL)-specific monoclonal antibody, 88B8, that abolishes the binding of LPL to GPIHBP1
Allan CM, et al.
Journal of Lipid Research, jlr-M070813 (2016)
G. Ottolina et al.
Biotechnology Letters, 14, 947-947 (1992)
Macrophage-mediated erosion of gamma irradiated poly (trimethylene carbonate) films
Bat E, et al.
Biomaterials, 30(22), 3652-3661 (2009)
Protocols
Lipoprotein lipase (LPL) hydrolyzes triglycerides associated with VLDL.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service