810282P
Avanti
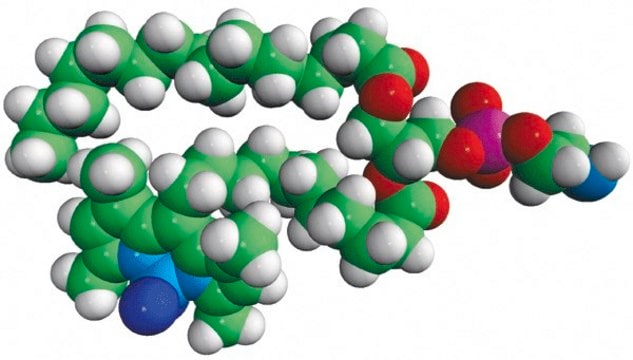
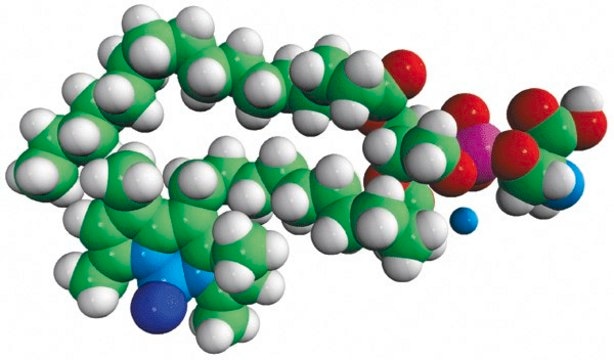
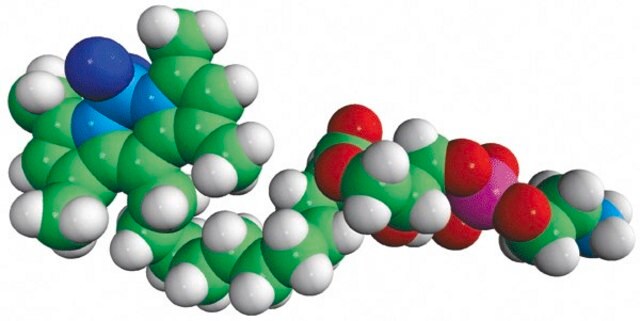
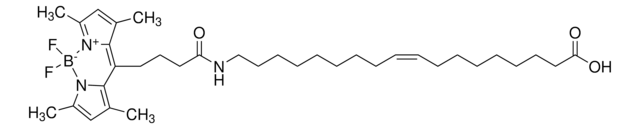
TopFluor™ PE
Avanti Research™ - A Croda Brand 810282P, powder
Synonym(s):
1-palmitoyl-2-(dipyrrometheneboron difluoride)undecanoyl-sn-glycero-3-phosphoethanolamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C45H77BF2N3O8P
CAS Number:
Molecular Weight:
867.89
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (810282P-1mg)
pkg of 5 × 1 mg (810282P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 810282P
shipped in
dry ice
storage temp.
−20°C
General description
Phosphoethanolamine (PHOS) is a precursor to phosphatidylcholine and phosphatidylethanolamine. The phosphatidylethanolamine (PE) is a zwitterionic phospholipid.
Biochem/physiol Actions
Phosphatidylethanolamine controls membrane fluidity in eukaryotic cells. Phosphoethanolamine (PHOS) has the ability to repress tumour growth both in vitro and in vivo.
Packaging
5 mL Amber Glass Screw Cap Vial (810282P-1mg)
5 mL Amber Glass Screw Cap Vial (810282P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
TopFluor is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lipid-protein interactions in membranes
Marsh D
The Membranes of Cells (1990)
Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells
Dawaliby R, et al.
Test, 3658-3667 (2016)
Phosphoethanolamine and the danger of unproven drugs
Ponde N, et al.
Ecancermedicalscience (2016)
Pei Qiao et al.
Biochemistry, 59(22), 2089-2099 (2020-05-07)
Activation of G-protein-gated inwardly rectifying potassium channels (Kir3.x) requires the direct binding of phosphorylated phosphatidylinositides (PIPs). Previous studies have established that PIP isoforms activate Kir channels to varying degrees and the binding affinity between PIPs and Kir3.2 appears to be
Lipid-protein interactions in membranes
Febs Letters, 268(2), 371-375 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![18:1 TopFluor™ PE 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[(dipyrromethaneboron difluoride)butanoyl], powder](/deepweb/assets/sigmaaldrich/product/structures/257/003/a0424abf-7aad-47b8-a731-c209a116d95c/640/a0424abf-7aad-47b8-a731-c209a116d95c.png)