86600
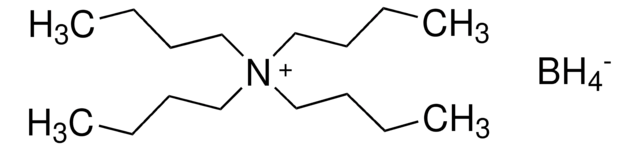
Tetraethylammonium borohydride
technical, ≥95% (T)
Synonym(s):
NSC 164898, NSC 76086, TEAB, Tetraethylammonium tetrahydroborate
About This Item
Recommended Products
grade
technical
Assay
≥95% (T)
form
crystals
reaction suitability
reagent type: reductant
mp
>230 °C
SMILES string
[BH4-].CC[N+](CC)(CC)CC
InChI
1S/C8H20N.BH4/c1-5-9(6-2,7-3)8-4;/h5-8H2,1-4H3;1H4/q+1;-1
InChI key
JFXPWTFDWBWOAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Reducing agent for the preparation of hydroxybaccatin III carbonate derivatives
- Reductant for the preparation of edge-bridged all-ferrous double cubane
- Reducing agent for the reduction of heterometal cubane iron/molybdenum cluster trigonally symmetrized with hydrotris(pyrazolyl)borate capping ligand
- Reactant for reactions with ruthenium phenanthroline carbonyl complexes yielding formyl complexes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3 - Water-react 2
Target Organs
Respiratory system
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service