56413
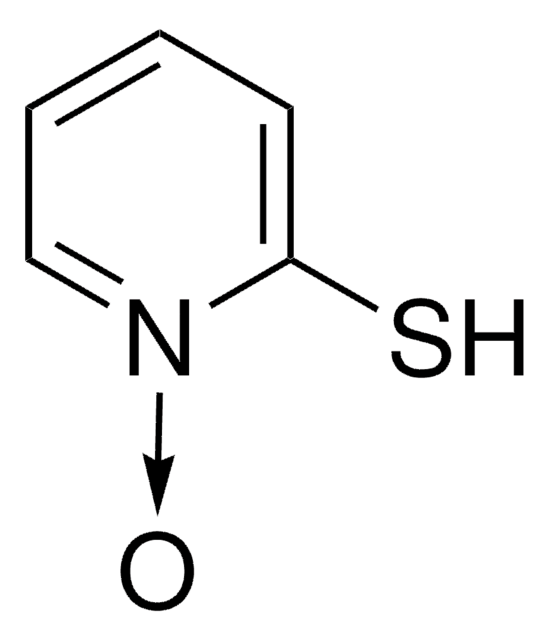
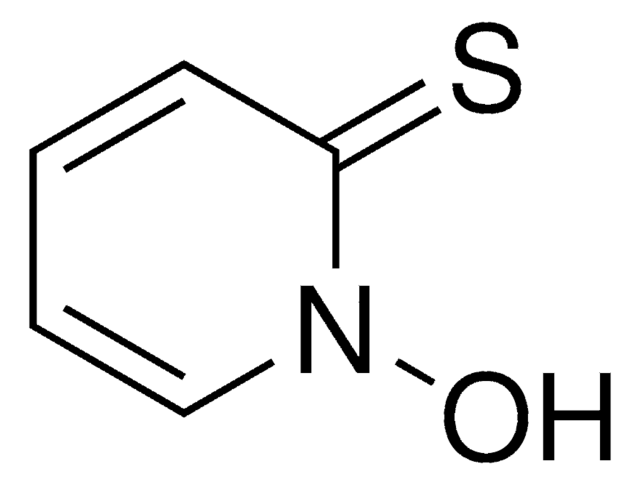
2-Pyridinol 1-oxide
≥98.0% (N)
Synonym(s):
1-Hydroxy-2-pyridone, 2-Hydroxypyridine 1-oxide, HOPO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO2
CAS Number:
Molecular Weight:
111.10
Beilstein:
1422545
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (N)
form
solid
mp
147-152 °C
SMILES string
Oc1cccc[n+]1[O-]
InChI
1S/C5H5NO2/c7-5-3-1-2-4-6(5)8/h1-4,7H
InChI key
JVHZMYAXZUIZKS-UHFFFAOYSA-N
Related Categories
General description
2-Pyridinol 1-oxide (HOPO) is a peptide coupling agent used as a subsititue for HOBt. It can form stable chelate complexes with transition metal ions.
Application
2-Pyridinol 1-oxide can be used:
- To fabricate gold electrodes for the electrochemical determination of europium using cyclic voltammetry.
- To prepare polymeric nickel (II) complex of 2-hydroxypyridine-N-oxide.
- In the synthesis of 1-methoxypyrid-4-one in the presence of sodium methoxide and methyl toluene-9-sulphonate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure and Behavior of Organic Analytical Reagents. Formation Constants of Transition Metal Complexes of 2-Hydroxypyridine-1-Oxide and 2-Mercaptopyridine-1-Oxide.
Sun PJ, et al.
Analytical Chemistry, 36(13), 2485-2488 (1964)
Laura Cruickshank et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 31(7), 623-627 (2015-07-15)
This work presents for the first time the electrochemical determination of europium using cyclic voltammetry at gold electrodes modified with 2-pyridinol-1-oxide. A well-defined oxidation peak was observed in cyclic voltammetry as a result of the oxidation of the europium at
Ali Younes et al.
Metallomics : integrated biometal science, 11(2), 496-507 (2019-01-16)
Uranium is widespread in the environment, resulting both from natural occurrences and anthropogenic activities. Its toxicity is mainly chemical rather than radiological. In the blood it is transported as uranyl UO22+ cation and forms complexes with small ligands like carbonates
875. N-oxides and related compounds. Part V. The tautomerism of 2-and 4-amino-and-hydroxy-pyridine 1-oxide.
Gardne JN & Katritzky AR.
Journal of the Chemical Society, 4375-4385 (1957)
Dong-Geon Lee et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 20(5), 752-756 (2019-01-11)
Porous carbonaceous anode materials have received considerable attention as an alternative anode material, however, there is a critical bottleneck as it suffers from a large irreversible specific capacity loss over several initial cycles owing to undesired surface reactions. In order
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service