442631
Coumarin 6
98%
Synonym(s):
3-(2-Benzothiazolyl)-7-(diethylamino)coumarin, 3-(2-Benzothiazolyl)-N,N-diethylumbelliferylamine
About This Item
Recommended Products
Assay
98%
form
solid
mp
208-210 °C (lit.)
λmax
444 nm
fluorescence
λem 505 nm in ethanol (Lasing peak 534 nm, lasing range 515 - 558 nm (DMSO), pump source XeCl (308 nm))
OLED Device Performance
ITO/Alq3:Coumarin 6/Mg:Ag
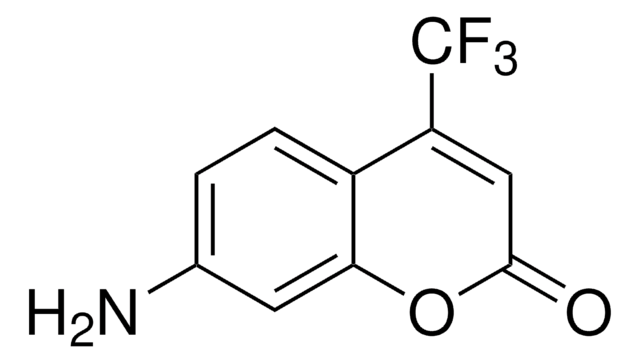
SMILES string
CCN(CC)c1ccc2C=C(C(=O)Oc2c1)c3nc4ccccc4s3
InChI
1S/C20H18N2O2S/c1-3-22(4-2)14-10-9-13-11-15(20(23)24-17(13)12-14)19-21-16-7-5-6-8-18(16)25-19/h5-12H,3-4H2,1-2H3
InChI key
VBVAVBCYMYWNOU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in block copolymer (BCP)-based micelle based drug delivery studies in glioma cell lines
- in combination with flufenamic acid (FA) based nanoprodrug uptake in glioma cells
- in poly(lactic-co-glycolic acid) (PLGA) based elvitegravir nanoprodrug uptake studies
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Micelle formation addresses low solubility in IV drug delivery, overcoming clinical limitations.
Micelle formation addresses low solubility in IV drug delivery, overcoming clinical limitations.
Micelle formation addresses low solubility in IV drug delivery, overcoming clinical limitations.
Micelle formation addresses low solubility in IV drug delivery, overcoming clinical limitations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service