431966
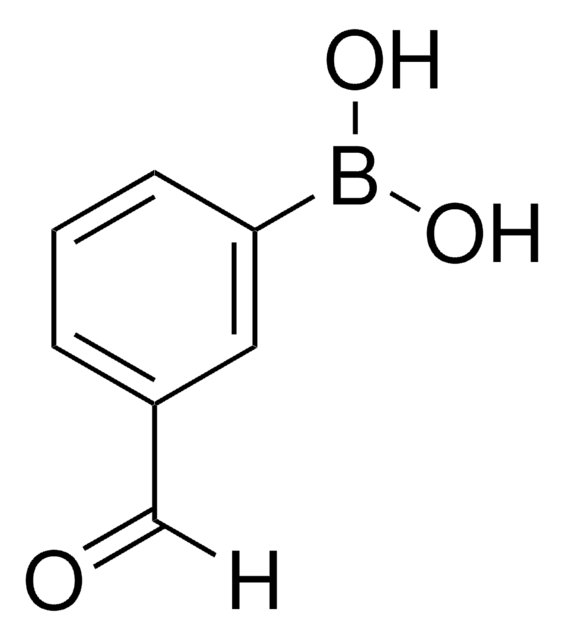
4-Formylphenylboronic acid
≥95.0%
Synonym(s):
4-(Dihydroxyboryl)benzaldehyde, 4-Boronobenzaldehyde, 4-Formylbenzeneboronic acid, p-Formylbenzeneboronic acid, p-Formylphenylboronic acid
About This Item
Recommended Products
Assay
≥95.0%
mp
237-242 °C (lit.)
SMILES string
OB(O)c1ccc(C=O)cc1
InChI
1S/C7H7BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5,10-11H
InChI key
VXWBQOJISHAKKM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service