360899
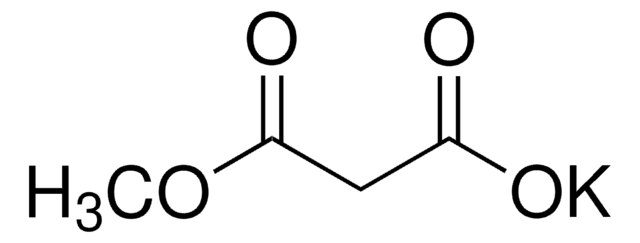
Ethyl potassium malonate
98%
Synonym(s):
Monoethyl malonate potassium salt, Potassium monoethyl malonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
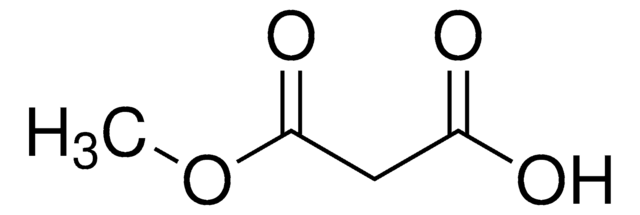
C2H5OCOCH2CO2K
CAS Number:
Molecular Weight:
170.20
Beilstein:
3721682
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
194 °C (dec.) (lit.)
functional group
ester
SMILES string
[K+].CCOC(=O)CC([O-])=O
InChI
1S/C5H8O4.K/c1-2-9-5(8)3-4(6)7;/h2-3H2,1H3,(H,6,7);/q;+1/p-1
InChI key
WVUCPRGADMCTBN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
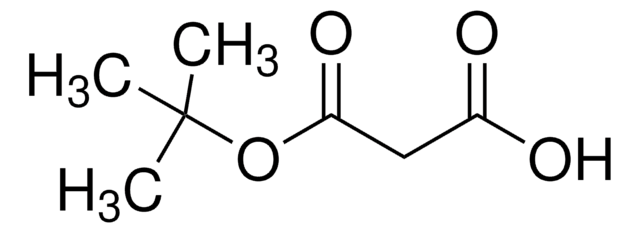
Ethyl potassium malonate (potassium ethyl malonate) reacts with aryl nitriles in the presence of zinc chloride and a catalytic amount of Hünig′s base to yield β-amino acrylates. Ethyl potassium malonate is formed as an intermediate during the synthesis of ethyl tert-butyl malonate.
Application
Ethyl potassium malonate (potassium ethyl malonate) may be used to generate (trimethylsilyl)ethyl malonate in situ, which can be acylated to prepare a variety of β-ketoesters or alkylidene malonates.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jae Hoon Lee et al.
The Journal of organic chemistry, 72(26), 10261-10263 (2007-12-01)
Reaction of aryl nitriles with potassium ethyl malonate in the presence of zinc chloride and a catalytic amount of Hünig's base provided beta-amino acrylates in moderate to good yield. Compared to the classical Blaise reaction, this reaction is safer (endothermic)
A process for the synthesis of ?-ketoesters using in-situ generated (trimethylsilyl) malonates
Wang X, et al.
Tetrahedron Letters, 35(50), 9323-9326 (1994)
Ethyl tert-Butyl Malonate.
Strube RE.
Organometallic Syntheses, 34-34 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service