All Photos(1)
About This Item
Linear Formula:
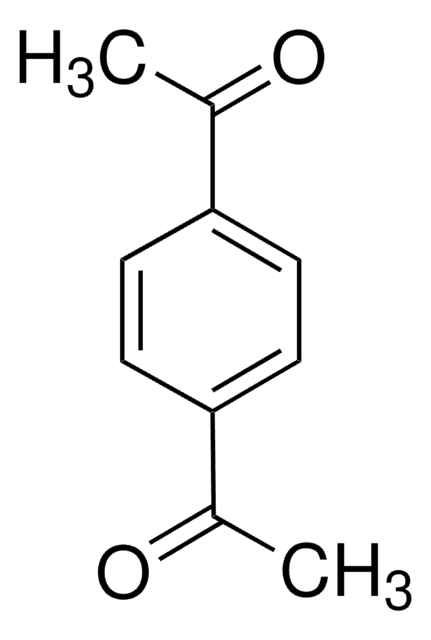
C6H4(COCH3)2
CAS Number:
Molecular Weight:
162.19
Beilstein:
1862907
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
110 °C/0.1 mmHg (lit.)
mp
39-41 °C (lit.)
solubility
dichloromethane: soluble 50 mg/mL, clear, colorless to yellow
fluorescence
λex 355 nm; λem 455 nm (Amine adducts)
functional group
ketone
SMILES string
CC(=O)c1ccccc1C(C)=O
InChI
1S/C10H10O2/c1-7(11)9-5-3-4-6-10(9)8(2)12/h3-6H,1-2H3
InChI key
LVQFKRXRTXCQCZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2-Diacetylbenzene, an aromatic hydrocarbon, is a protein-reactive γ-diketone metabolite of the neurotoxic solvent 1,2-diethylbenzene.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Desire Tshala-Katumbay et al.
Toxicological sciences : an official journal of the Society of Toxicology, 107(2), 482-489 (2008-11-27)
Neuroprotein changes in the spinal cord of rodents with aliphatic gamma-diketone axonopathy induced by 2,5-hexanedione (2,5-HD) are compared with those reported previously in aromatic gamma-diketone-like axonopathy induced by 1,2-diacetylbenzene (1,2-DAB). Sprague-Dawley rats were treated intraperitoneally with 500 mg/kg/day 2,5-HD, equimolar
Xin-Jun Yu et al.
Molecules (Basel, Switzerland), 25(6) (2020-03-18)
In the present study, a pyridoxal-5'-phosphate (PLP)-dependent L-aspartate-α-decarboxylase from Tribolium castaneum (TcPanD) was selected for protein engineering to efficiently produce β-alanine. A mutant TcPanD-R98H/K305S with a 2.45-fold higher activity than the wide type was selected through error-prone PCR, site-saturation mutagenesis
Chang-Guo Zhan et al.
Journal of the American Chemical Society, 124(11), 2744-2752 (2002-03-14)
We report the first computational study of the chromophores responsible for the chromogenic effects of aromatic neurotoxicants containing a 1,2-diacetyl moiety in their oxidation metabolites. A series of ab initio electronic structure calculations was performed on two representative aromatic compounds
Mohammad I Sabri et al.
Neurochemical research, 32(12), 2152-2159 (2007-06-20)
The aromatic hydrocarbon 1,2-diacetylbenzene (1,2-DAB) is a protein-reactive gamma-diketone metabolite of the neurotoxic solvent 1,2-diethylbenzene (1,2-DEB). The effect of neurotoxic 1,2-DAB and its non-neurotoxic isomer 1,3-DAB has been studied on motor proteins and cytoskeletal proteins of rat spinal cord (SC).
Min-Sun Kim et al.
Chemico-biological interactions, 194(2-3), 139-147 (2011-10-25)
1,2-Diacetylbenzene (DAB) is a neurotoxic minor metabolite of 1,2-diethylbenzene or naphthalene reaction product with OH radical. DAB causes central and peripheral neuropathies that lead to motor neuronal deficits. However, the potent effects and molecular mechanisms of DAB on neural progenitor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service