All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H4ClNO
CAS Number:
Molecular Weight:
129.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
160 °C (dec.) (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
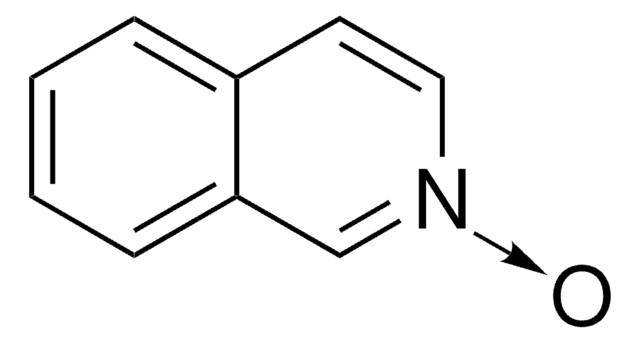
[O-][n+]1ccc(Cl)cc1
InChI
1S/C5H4ClNO/c6-5-1-3-7(8)4-2-5/h1-4H
InChI key
DPJVRASYWYOFSJ-UHFFFAOYSA-N
General description
4-Chloropyridine N-oxide forms complexes with uranyl chloride and has been investigated by IR spectra, electronic spectra, molar conductivity and magnetic susceptibility measurements.
Application
4-Chloropyridine N-oxide was used as oxygen source in the preparation of [(N,N′-bis(2-methyl-2-mercaptopropane)-1,5-diazacyclooctane)Fe]2O complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ghezai Musie et al.
Inorganic chemistry, 37(16), 4086-4093 (2001-10-24)
The (&mgr;-oxo)diiron(III) complexes of N,N'-bis(2-methyl-2-mercaptopropane)-1,5-diazacyclooctane (H(2)bme-daco) and N,N'-bis(mercaptoethyl)-1,5-diazacyclooctane (H(2)bme-daco) ligands present uncommon examples of well-characterized (&mgr;-oxo)diiron(III) species in the presence of sulfur donors. Whereas the reaction of molecular oxygen with a solution of diiron(II) complex, [(bme-daco)Fe](2), affords slow formation of
Uranyl chloride complexes of pyridine-N-oxide and 4-substituted pyridine-N-oxides: Infra-red spectra, electronic spectra and magnetic susceptibility studies.
Balakrishnan PV, et al.
J. Inorg. Nucl. Chem., 28(2), 537-541 (1966)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service