PHR1521
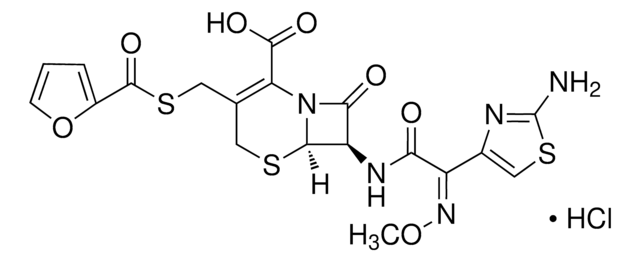
Ceftiofur Sodium
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
Sodium Ceftiofur
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to USP 1098165
Famiglia di API
ceftiofur
CdA
current certificate can be downloaded
Confezionamento
ampule of 1 g
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
-10 to -25°C
Descrizione generale
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Applicazioni
Nota a piè di pagina
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Resp. Sens. 1 - Skin Sens. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.