424633
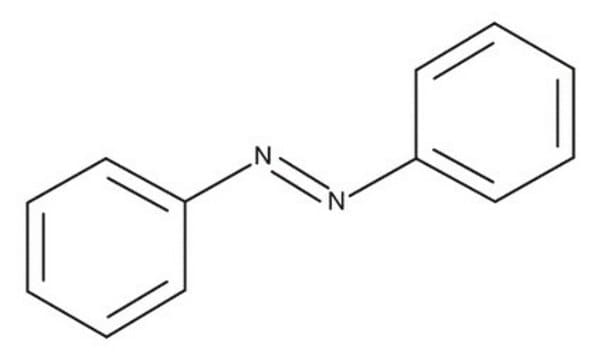
Azobenzene
98%
Sinonimo/i:
1,2-Diphenyldiazene; Diphenyldiazene
About This Item
Prodotti consigliati
Tensione di vapore
1 mmHg ( 104 °C)
Livello qualitativo
Saggio
98%
Forma fisica
powder or crystals
Temp. autoaccensione
890 °F
P. eboll.
293 °C (lit.)
Punto di fusione
65-68 °C (lit.)
Densità
1.09 g/mL at 25 °C (lit.)
applicazioni
diagnostic assay manufacturing
hematology
histology
Temperatura di conservazione
room temp
Stringa SMILE
c1ccc(cc1)\N=N\c2ccccc2
InChI
1S/C12H10N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H/b14-13+
DMLAVOWQYNRWNQ-BUHFOSPRSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Muta. 2 - STOT RE 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
212.0 °F - closed cup
Punto d’infiammabilità (°C)
100.0 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.