367796
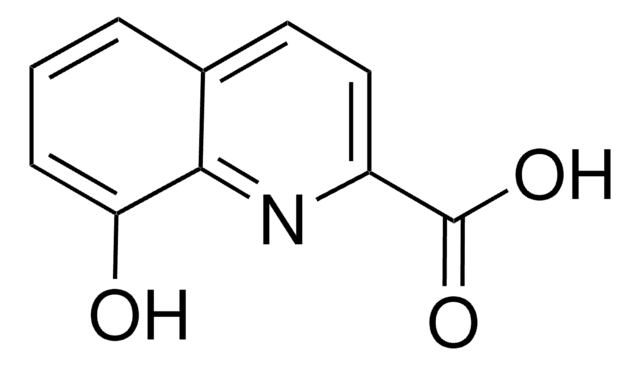
8-Quinolinecarboxylic acid
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H7NO2
Numero CAS:
Peso molecolare:
173.17
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
183-185 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
OC(=O)c1cccc2cccnc12
InChI
1S/C10H7NO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6H,(H,12,13)
QRDZFPUVLYEQTA-UHFFFAOYSA-N
Descrizione generale
Herbicide 8-quinolinecarboxylic acid and its removal from aqueous solution using sodium montmorillonite, acidic montmorillonite and organo-acidic montmorillonite has been reported.
Applicazioni
8-Quinolinecarboxylic acid may be used in the synthesis of:
- novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives
- chiral 1,2,3,4-tetrahydroquinolinyl-oxazoline compounds, used as ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones
- chiral quinolinyl-oxazoline compounds, used as ligands for Cu(II) catalyzed asymmetric cyclopropanation
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
S López-Bernabeu et al.
Chemosphere, 144, 982-988 (2015-10-05)
The effect of the electrochemical treatment (potentiostatic treatment in a filter-press electrochemical cell) on the adsorption capacity of an activated carbon cloth (ACC) was analyzed in relation with the removal of 8-quinolinecarboxylic acid pollutant from water. The adsorption capacity of
Chiral quinolinyl-oxazolines as ligands for copper (I)-catalyzed asymmetric cyclopropanation.
Wu X-Y, et al.
Tetrahedron Asymmetry, 9(23), 4143-4150 (1998)
Chiral 1, 2, 3, 4-tetrahydroquinolinyl-oxazoline ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones.
Zhou Y-B, et al.
Tetrahedron Asymmetry, 13(5), 469-473 (2002)
Barbara Machura et al.
Dalton transactions (Cambridge, England : 2003), 42(24), 8827-8837 (2013-05-04)
Six novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives were prepared in good yields. Relying on the experimental conditions, compounds with two chelate ligands [ReOCl(iqc)2]·MeOH (1), [ReO(OMe)(iqc)2] (2), [ReO(OMe)(mqc)2] (3) and [ReO(OMe)(8-qc)2] (4) and compounds incorporating one bidentate
M Mekhloufi et al.
Environmental monitoring and assessment, 185(12), 10365-10375 (2013-08-09)
Sodium montmorillonite (Na-M), acidic montmorillonite (H-M), and organo-acidic montmorillonite (Org-H-M) were applied to remove the herbicide 8-quinolinecarboxylic acid (8-QCA). The montmorillonites containing adsorbed 8-QCA were investigated by transmission electron microscopy, FT-IR spectroscopy, X-ray diffraction analysis, X-ray fluorescence thermogravimetric analysis, and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.