165336
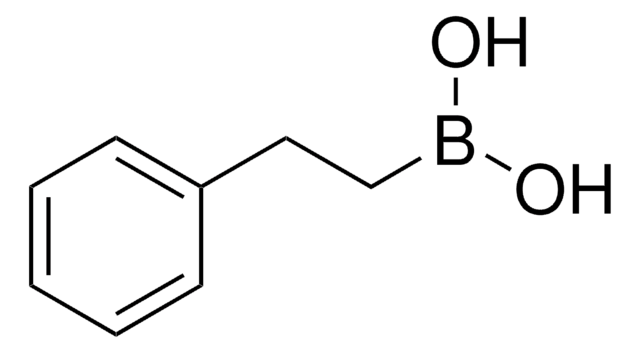
Methylboronic acid
97%
Sinonimo/i:
Methaneboronic acid
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
91-94 °C (lit.)
Stringa SMILE
CB(O)O
InChI
1S/CH5BO2/c1-2(3)4/h3-4H,1H3
KTMKRRPZPWUYKK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- In the palladium-catalyzed Stille and Suzuki-Miyaura cross-couplings.
- In the microwave-heated heterogeneous palladium (Pd)-catalytized reactions.
- In ruthenium (Ru)-catalyzed silylation reactions
- To prepare bis(aminotropone) titanium (Ti) catalysts for ethylene polymerizations.
- In the enantioselective asymmetric bromoaminocyclization and bromoaminocyclization using amino-thiocarbamate catalysts.
- To prepare common building blocks for pharmaceuticals and agrochemicals.
- To prepare chrysin analogs by Suzuki-Miyaura coupling reactions.
- To prepare casein kinase I inhibitors.
- In the divergent C-H functionalizations directed by sulfonamide pharmacophores in drug discovery.
- In the synthesis of unsymmetrical monosulfides from disulfides via copper-catalyzed coupling with boronic acids.
- In a palladium-catalyzed coupling with enol tosylates.
- For derivatizing many carbohydrates and biologically active compounds for GLC analysis.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)






![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)