SML1308
KT195
≥98% (HPLC)
Synonyme(s) :
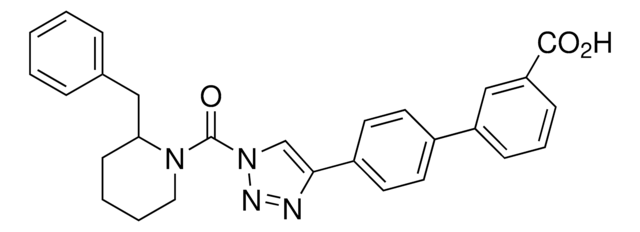
[4-(4′-Methoxy[1,1′-biphenyl]-4-yl)-1H-1,2,3-triazol-1-yl](2-phenyl-1-piperidinyl)-methanone
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98% (HPLC)
Forme
powder
Couleur
white to beige
Solubilité
DMSO: 5 mg/mL, clear (warmed)
Température de stockage
2-8°C
Actions biochimiques/physiologiques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral - Aquatic Chronic 4
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Contenu apparenté
The aim of the Cravatt research group is to understand the roles that mammalian enzymes play in physiological and pathological processes and to use this knowledge to identify novel therapeutic targets for the treatment of human disease. To achieve these goals, they develop and apply new technologies that bridge the fields of chemistry and biology, ascribing to the philosophy that the most significant biomedical problems require creative multidisciplinary approaches for their solution. The group's technological innovations address fundamental challenges in systems biology that are beyond the scope of contemporary methods. For instance, enzymes are tightly regulated by post-translational events in vivo, meaning that their activity may not correlate with expression as measured by standard genomic and proteomic approaches. Considering that it is an enzyme's activity, rather than abundance that ultimately dictates its role in cell physiology and pathology, the Cravatt group has introduced a set of proteomic technologies that directly measures this parameter. These activity-based protein profiling (ABPP) methods exploit the power of chemistry to engender new tools and assays for the global analysis of enzyme activities. The enzyme activity profiles generated by ABPP constitute unique molecular portraits of cells and tissues that illuminate how metabolic and signaling networks are regulated in vivo. Additionally, by evaluating enzymes based on functional properties rather than mere abundance, ABPP acquires high-content proteomic information that is enriched in novel markers and targets for the diagnosis and treatment of human disease.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique