C8271
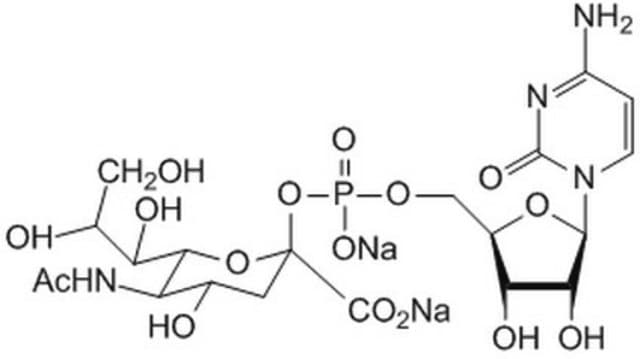
Cytidine-5′-monophospho-N-acetylneuraminic acid sodium salt
≥85% (HPLC)
Synonyme(s) :
CMP-NeuAc
About This Item
Produits recommandés
Source biologique
synthetic (organic)
Pureté
≥85% (HPLC)
Forme
powder
Température de stockage
−20°C
Chaîne SMILES
[Na+].CC(=O)N[C@@H]1[C@@H](O)C[C@@](O[C@@H]1[C@H](O)[C@H](O)CO)(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(N)=NC3=O)C(O)=O
InChI
1S/C20H31N4O16P.Na/c1-7(26)22-12-8(27)4-20(18(32)33,39-16(12)13(29)9(28)5-25)40-41(35,36)37-6-10-14(30)15(31)17(38-10)24-3-2-11(21)23-19(24)34;/h2-3,8-10,12-17,25,27-31H,4-6H2,1H3,(H,22,26)(H,32,33)(H,35,36)(H2,21,23,34);/q;+1/p-1/t8-,9+,10+,12+,13+,14+,15+,16?,17+,20+;/m0./s1
Clé InChI
VFRHSOGUONIUOR-CTFMUGKASA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- as a standard in high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) for nucleotide sugar analysis in Joubert syndrome type 10 (JBTS10) patient cells and control skin fibroblasts,
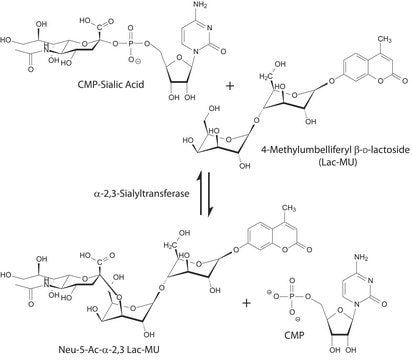
- As a substrate for the enzymatic sialylation of G2 glycoforms, resialylation assay,
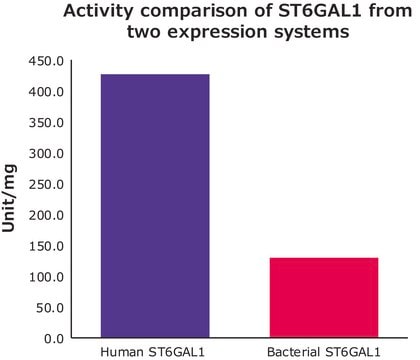
- in in-vitro sialyltransferase assay
Actions biochimiques/physiologiques
Notes préparatoires
CMP-NAN is very acid-labile.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
LC-MS/MS method quantifies similar polar nucleotide activated sugars using Supel™ Carbon LC column for simultaneous analysis.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Understand sialic acid structure, function, signaling, and modifications. Easily find products for sialic acid research.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique