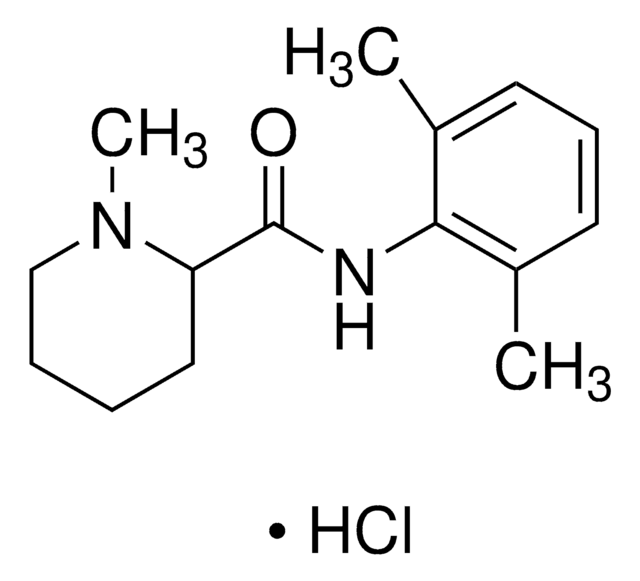
PHR1257
Lidocaine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
Lidocaine hydrochloride monohydrate, 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate, Lignocaine hydrochloride monohydrate, Xylocaine hydrochloride monohydrate
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to BP 214
traceable to Ph. Eur. L0600000
traceable to USP 1366013
Famille d'API
lidocaine
CofA (certificat d'analyse)
current certificate can be downloaded
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-30°C
Chaîne SMILES
Cl[H].[H]O[H].CCN(CC)CC(=O)Nc1c(C)cccc1C
InChI
1S/C14H22N2O.ClH.H2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H;1H2
Clé InChI
YECIFGHRMFEPJK-UHFFFAOYSA-N
Informations sur le gène
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Lidocaine hydrochloride is an amide type local anaesthetic that belongs to the class of 1b antiarrhythmics. It is used for regional nerve blocks and infiltrative administration of anaesthesia. It prevents the entry of sodium ions into nerve endings, at the site of pain, and disrupts the electrical signal from reaching the brain.
Application
- Sensitive determination of lidocaine hydrochloride and cetylpyridinium chloride in their binary mixtures in different pharmaceutical formulations by three spectrophotometric-based methods
- Simultaneous analysis of aminoacridine hydrochloride and lidocaine hydrochloride in bulk powder and pharmaceutical formulation by high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC)-densitometric methods
- Quantification of ceftriaxone sodium and lidocaine HCl in human plasma samples by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS)
- Quantitative analysis of lidocaine hydrochloride and miramistin in a wound healing gel sample by high-performance liquid chromatography (HPLC) combined with UV detection
- Development of a method based on liquid–liquid extraction of nifedipine and lidocaine hydrochloride from human plasma samples for their subsequent analysis by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)
- Dispersive liquid-liquid microextraction (DLLME) followed by attenuated total reflectance-Fourier transform infrared measurement of dry films for the determination of lidocaine hydrochloride in human urine sample
Actions biochimiques/physiologiques
Remarque sur l'analyse
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique