P0532
Phthaldialdehyde Reagent
Solution Complete
Synonyme(s) :
OPA
About This Item
Produits recommandés
Niveau de qualité
Température de stockage
2-8°C
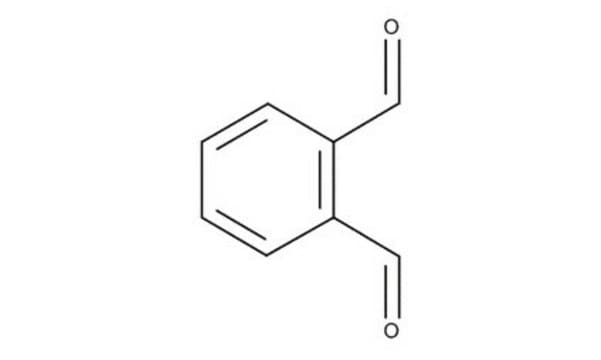
Chaîne SMILES
[H]C(=O)c1ccccc1C([H])=O
InChI
1S/C8H6O2/c9-5-7-3-1-2-4-8(7)6-10/h1-6H
Clé InChI
ZWLUXSQADUDCSB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Aquatic Chronic 2 - Eye Dam. 1 - Met. Corr. 1 - Repr. 1B - Skin Corr. 1B - Skin Sens. 1
Classe de danger pour l'eau (WGK)
WGK 3
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique