70372
1,4-Naphthoquinone
purum, ≥96.5% (HPLC)
Synonyme(s) :
α-Naphthoquinone
About This Item
Produits recommandés
Qualité
purum
Niveau de qualité
Pureté
≥96.5% (HPLC)
Forme
powder
Pf
119-122 °C (lit.)
120-124 °C
Chaîne SMILES
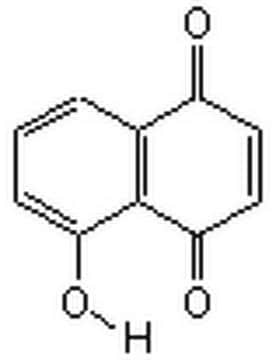
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
Clé InChI
FRASJONUBLZVQX-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
It can be used to synthesize:
- 3,3-Disubstituted oxindoles via asymmetric Michael addition to oxindole.
- Bioactive isoindolines via asymmetric 1,3-dipolar cycloaddition to azomethine ylides generated in situ from aldehydes and diethyl aminomalonate.
- α,α-Difluoro-β-hydroxy ketone via ‘on water′ catalyst-free Mukaiyama-aldol reaction with difluoroenoxysilane.
- 2-Hydroxy-3-anilino-1,4-naphthoquinone, which shows potent in vivo antimalarial activity.
Additional appilcation include:
- As an arylation reagent for the α-arylation of aldehydes.
- As a starting material in the multi-step synthesis of benz[f]indole-4,9-diones.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
285.8 °F
Point d'éclair (°C)
141 °C
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique