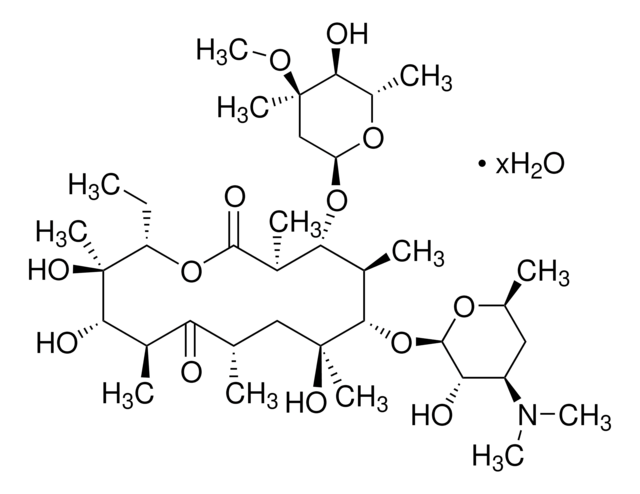
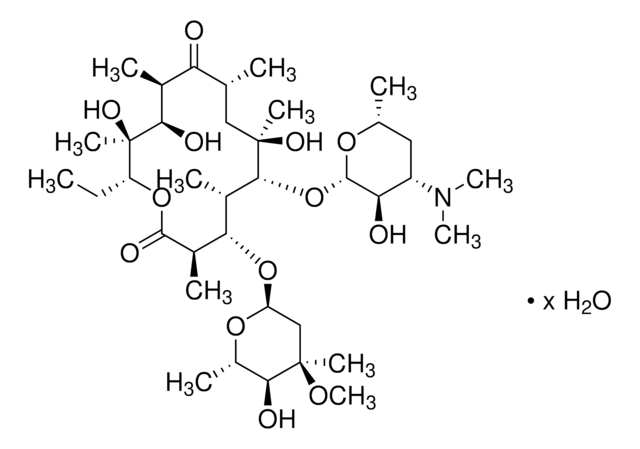
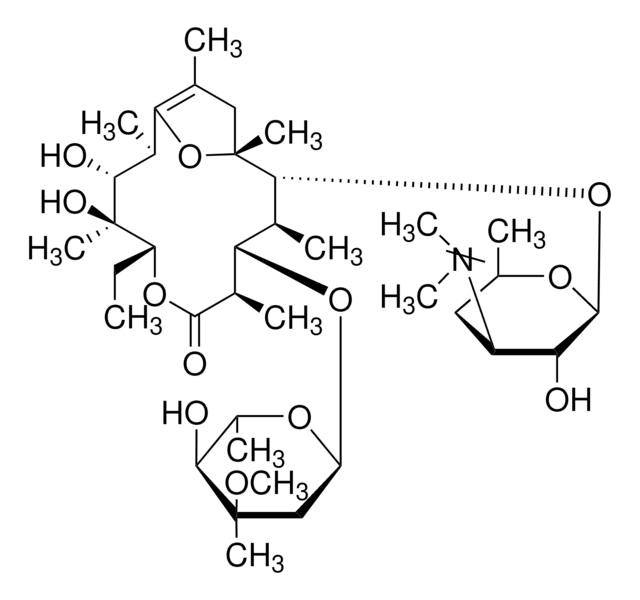
45674
Erythromycin
tested according to Ph. Eur.
Synonyme(s) :
Erythromycinum
About This Item
Produits recommandés
Agence
EPA 1694
USP/NF
tested according to Ph. Eur.
Niveau de qualité
Forme
solid
Couleur
white to faint yellow
Solubilité
H2O: soluble 2 mg/mL
acetone: freely soluble
acetonitrile: freely soluble
alcohol: soluble
amyl acetate: moderately soluble
chloroform: soluble
diethyl ether: soluble
ethyl acetate: freely soluble
Spectre d'activité de l'antibiotique
Gram-negative bacteria
Gram-positive bacteria
Application(s)
environmental
Mode d’action
protein synthesis | interferes
Chaîne SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
Clé InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Informations sur le gène
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Conditionnement
Attention
Notes préparatoires
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique