PF024
MMP-9, Active, Human, Recombinant
Synonyme(s) :
Gelatinase B, 83 kDa Gelatinase, Matrix Metalloproteinase 9, Matrix Metalloproteinase 9, Gelatinase B, 83 kDa Gelatinase
About This Item
Produits recommandés
Pureté
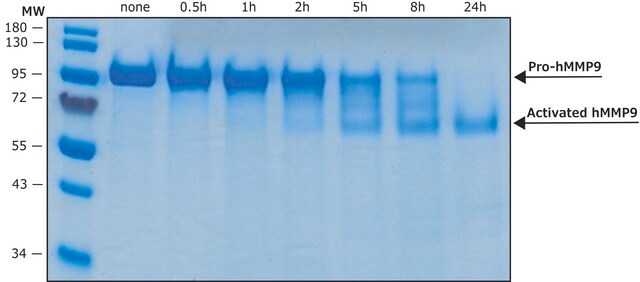
≥90% (SDS-PAGE)
Niveau de qualité
Forme
liquid
Activité spécifique
≥8.0 ΔA405/h-μg protein (thiopeptide hydrolysis assay)
Ne contient pas
preservative
Fabricant/nom de marque
Calbiochem®
Conditions de stockage
OK to freeze
avoid repeated freeze/thaw cycles
Conditions d'expédition
wet ice
Température de stockage
−70°C
Description générale
Regulation of MMP activity can occur at the level of gene expression, including transcription and translation, level of activation, or at the level of inhibition by TIMPs. Thus, perturbations at any of these points can theoretically lead to alterations in ECM turnover. Expression is under tight control by pro- and anti-inflammatory cytokines and/or growth factors and, once produced the enzymes are usually secreted as inactive zymograms. Upon activation (removal of the inhibitory propeptide region of the molecules) MMPs are subject to control by locally produced TIMPs. All MMPs can be activated in vitro with organomercurial compounds (e.g. 4-aminophenylmercuric acetate), but the agents responsible for the physiological activation of all MMPs have not been clearly defined. Numerous studies indicate that members of the MMP family have the ability to activate one another. The activation of the MMPs in vivo is likely to be a critical step in terms of their biological behavior, because it is this activation that will tip the balance in favor of ECM degradation. The hallmark of diseases involving MMPs appear to be stoichiometric imbalance between active MMPs and TIMPs, leading to excessive tissue disruption and often degradation. Determination of the mechanisms that control this imbalance may open up some important therapeutic options of specific enzyme inhibitors.
Conditionnement
Avertissement
Forme physique
Reconstitution
Autres remarques
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J. Neurochem.67, 251.
Xia, T., et al. 1996. Biochim. Biophys. Acta1293, 259.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta1251, 99.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 217.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 484.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
Informations légales
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique