8.55019
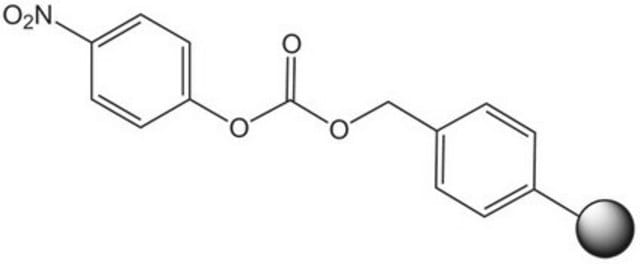
p-Nitrophenyl carbonate Wang resin
Novabiochem®
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352005
Produits recommandés
Niveau de qualité
Gamme de produits
Novabiochem®
Forme
beads
Capacité de réaction
reaction type: Fmoc solid-phase peptide synthesis
Pertinence de la réaction
reactivity: amine reactive
Fabricant/nom de marque
Novabiochem®
Application(s)
peptide synthesis
Température de stockage
15-25°C
Description générale
Resin-bound p-nitrophenyl carbonate esters react readily with amines to provide the corresponding resin-bound carbamate. Leznoff [1] was the first to demonstrate the utility of such resins in solid phase synthesis with the preparation of mono-acylated diamines from symmetrical diamines. Similarly, Dressman, et al. [2] have used carbamate linked resin-bound amino acid amides to make hydantoins via a base-mediated cyclization/cleavage strategy. Amines linked to p-nitrophenyl carbonate Wang resin possess similar chemical properties to methoxybenzyloxycarbonyl protected amines. The resin-bound carbamate is, therefore, cleaved with TFA or hydrogenolysis to afford the free amine [3]. Alternatively, cleavage by reduction with lithium aluminium hydride can be used to generate N-methylamines [4], and Raju & Kogan [5] have utilized this resin to prepare sulfonamides by acylation of immobilized arylamines and cleavage with LiOH or NaOMe. An analogous p-nitrophenyl carbonate resin prepared from NovaSyn TGA resin was employed as a support for the solid phase immobilization of amidines [6]. This resin was also used to synthesize polyamines [7, 8], and to immobilize indoles [9] and guanidinylating reagents [10]. Loading of this resin can be quantified by measuring the release of p-nitrophenolate [11].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Liaison
Replaces: 01-64-0123
Remarque sur l'analyse
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Informations légales
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique