07-1829
Anti-phospho-NF-kB (p100) (Ser707) Antibody
from rabbit, purified by affinity chromatography
Synonyme(s) :
Nuclear factor NF-kappa-B p100 subunit, DNA-binding factor KBF2, H2TF1, Lymphocyte translocation chromosome 10 protein, Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2, Oncogene Lyt-10, Lyt10, Nuclear factor NF-kappa-B p52 subunit
About This Item
Produits recommandés
Source biologique
rabbit
Niveau de qualité
Forme d'anticorps
affinity isolated antibody
Type de produit anticorps
primary antibodies
Clone
polyclonal
Produit purifié par
affinity chromatography
Espèces réactives
human
Technique(s)
dot blot: suitable
inhibition assay: suitable (peptide)
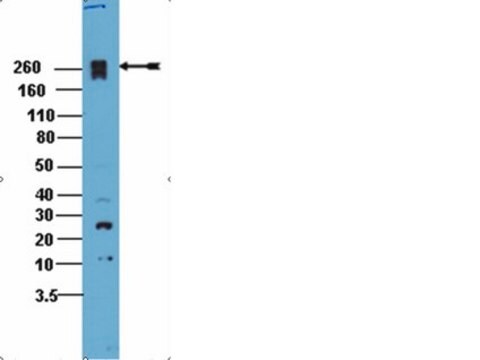
western blot: suitable
Numéro d'accès NCBI
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Modification post-traductionnelle de la cible
phosphorylation (pSer707)
Informations sur le gène
human ... NFKB1(4790)
Description générale
Spécificité
Immunogène
Application
Epigenetics & Nuclear Function
Transcription Factors
Qualité
Description de la cible
Forme physique
Stockage et stabilité
Remarque sur l'analyse
Phosphorylated and non-phosphorylated NF-kB peptides
Clause de non-responsabilité
Not finding the right product?
Try our Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique